Question
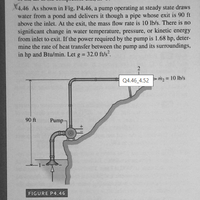
Transcribed Image Text:X4.46 As shown in Fig. P4.46, a pump operating at steady state draws
water from a pond and delivers it though a pipe whose exit is 90 ft
above the inlet. At the exit, the mass flow rate is 10 lb/s. There is no
significant change in water temperature, pressure, or kinetic energy
from inlet to exit. If the power required by the pump is 1.68 hp, deter-
mine the rate of heat transfer between the pump and its surroundings,
in hp and Btu/min. Let g = 32.0 ft/s.
Q4.46_4.52 m2 = 10 lb/s
90 ft
Pump-
FIGURE P4.46
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- question 4barrow_forwardWhile constant total pressure = 4.5 atm, an ideal gas grows from 500ml to 900ml. After, the heat flows out of the gas at constant volume. Pressure and temperature may drop until the temperature reaches its original value. During this process, what's the total work done by this gas (answer in J(? please use convsersion: 1atm=1.013x105 Paarrow_forwardA gas is compressed inside a cylinder. An average force of 67 N acts to move the piston 4.4 m. During the compression, 82 J of heat are conducted away from the gas. What is the change in internal energy of the gas?arrow_forward
- A piston-cylinder assembly contains 0.7 lb of air initially at a pressure of 30 lbf/in² and a temperature of 100°F. The air is heated at constant pressure until its volume is doubled. Assume the ideal gas model with constant specific heat ratio, k = 1.4. Determine the work and heat transfer, in Btu.arrow_forwardAn average force of 64 N acts to move the piston 0.9 m. During the compression, 2 J of heat are conducted away from the gas. What is the change in internal energy (in J) of the gas?arrow_forward
arrow_back_ios
arrow_forward_ios