
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
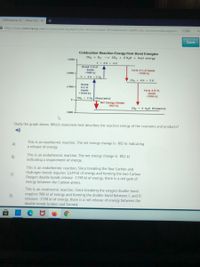
Study the graph. Which statement best describes the reaction energy of the reactants and products?
Transcribed Image Text:USATestprep, LLC- Online Stat X
+
A https://www.usatestprep.com/modules/test/tq.php?testid=9838&strandid=4935&element%=&difficulty=assessment&assignmen
110%
Save
Combustion Reaction Energy from Bond Energies
--> CO2 + 2 H20 + heat energy
CH4 + 02
+3000 -
C+ 4H +40
Break 2 0=0
bonds
988 kj
Form 2 C=0 bonds
-1598 kj
+2000 -
C + 4H + 2 02
Co, + 4 H 20
Break
+1000-
4 C-H
Form 4 0-H-
bonds
+1644 kj
bonds
-1836 kj
CH, + 2 02 (Reactants)
Net Energy Change
-802 kj
CO, + 2 H20 (Products)
-1000
Study the graph above. Which statement best describes the reaction energy of the reactants and products?
This is an exothermic reaction. The net energy change is -802 kl indicating
a release of energy.
A)
This is an endothermic reaction. The net energy change is -802 kJ
indicating a requirement of energy.
B)
This is an endothermic reaction. Since breaking the four Carbon and
Hydrogen bonds requires 1,644 kJ of energy and forming the two Carbon
Oxygen double bonds release -1598 kJ of energy, there is a net gain of
energy between the Carbon atoms.
C)
This is an exothermic reaction. Since breaking the oxygen double bond
requires 988 kJ of energy and forming the double bond between C and O
releases -1598 kJ of energy, there is a net release of energy between the
double bonds broken and formed.
D)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements is correct if a reaction is carried out at constant pressure? The heat change is equal to the change in temperature. The reaction is likely to be exothermic. The reaction is likely to be endothermic. The heat change is equal to the enthalpy change.arrow_forwardA balloon is filled with two gases that undergo a reaction. The temperature inside the balloonincreases whereas the volume of the balloon decreases. What can be said about the reaction?a) The reaction is exothermic, and the sign of w is +b) The reaction is endothermic, and the sign of w is —c) The reaction is exothermic, and the sign of w is —d) The reaction is endothermic, and the sign of w is +e) Not enough informationarrow_forwardA student is planning to determine the specific heat capacity of iron. To do this experiment the student will need to perform the following procedures: Step 1 :Measure the mass of the iron sample. Step 2: Measure the initial temperature of a known volume of water. Step 3: Heat the iron sample. Step 4: Place the iron sample in the water Step 5: ? What is Step 5 in the experiment?arrow_forward
- A chemist measures the energy change AH during the following reaction: 2 HgO(s) → 2 Hg(1) + O₂(g) Use the information to answer the following questions. This reaction is... Suppose 24.4 g of HgO react. Will any heat be released or absorbed? AH= 182. kJ If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits. O endothermic. O exothermic. O Yes, absorbed. O Yes, released. O No. x10 Xarrow_forwardA chemist measures the energy change AH during the following reaction: Cl₂(g) + H₂(g) → 2 HCl(g) Use the information to answer the following questions. This reaction is... Suppose 14.7 of Cl₂ react. g Will any heat be released or absorbed? ΔΗ = - 184. kJ If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits. endothermic. O exothermic. O Yes, absorbed. Yes, released. O No. ០៤ kJ ☐x10 Xarrow_forwardAll chemical reactions that are used as fuel must... O Have products higher in energy than reactants. O Have products lower in energy than réactant. O Have products with the same energy as the reactants.arrow_forward
- does adding temperature to a chemical reaction favor the reaction endothermically?arrow_forwardThe energy of an exothermic reaction is released into the environment O consumed in the reaction converted to another form absorbed by the reactionarrow_forwardSo,i do not understand nothing. On how to this. Please explain with steps,and also, can you mark how many times it went up and down,the limit is 8 steps going up. Thanksarrow_forward
- Please answer 9d and 9earrow_forwardConsider the reaction 2 H, + 0, — 2H,О AHrxn -484 kJ Which answer best describes the transfer of heat that occurs when 1.50 mol H, reacts? 363 kJ released 363 kJ absorbed 484 kJ released 726 kJ absorbed 726 kJ released 484 kJ absorbedarrow_forwardA chemist measures the energy change AH during the following reaction: Cl₂(g) + H₂(g) → 2 HC1 (g) AH=184. kJ Use the information to answer the following questions. endothermic. This reaction is... 0 exothermic. Suppose 28.0 g of Cl₂ react. Yes, absorbed. Yes, released. Will any heat be released or absorbed? No. If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits. ☐kJ x10 X ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY