
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
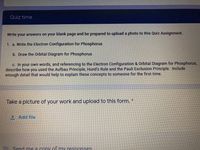
Transcribed Image Text:Quiz time
Write your answers on your blank page and be prepared to upload a photo to this Quiz Assignment.
1. a. Write the Electron Configuration for Phosphorus
b. Draw the Orbital Diagram for Phosphorus
c. In your own words, and referencing to the Electron Configuration & Orbital Diagram for Phosphorus,
describe how you used the Aufbau Principle, Hund's Rule and the Pauli Exclusion Principle. Include
enough detail that would help to explain these concepts to someone for the first time.
Take a picture of your work and upload to this form. *
1 Add file
Send me a cony of my responses
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemistryarrow_forwardWhich statement regarding the Bohr model of the atom is true?a. An element produces a continuous spectrum.b. Electrons lose quantized amounts of energy when moving to the ground state.c. Electrons can be anywhere, even between energy levels.d. Light is emitted when an electron moves from the ground state to an excited state.e. Electrons in the lowest energy level are in an excited state.arrow_forwarda. What is the maximum number of electrons that can go into the first shell? The second shell?b. How many electrons are present in an atom with its 1s, 2s and 2p subshells completely filled?c. How many subshells are in the 3rd shell?d. How many orbitals are there in the third principal energy level?arrow_forward
- 18- Which statement regarding the Bohr model of the atom is correct? A. Energy levels are electrons orbiting the nucleus are continuous B. As electrons get further away from the nucleus their energy levels decrease C. Energy levels of electrons orbiting the nucleus is quantized D. Electron energies around the nucleus are positive in valuearrow_forwardWhich of these elements has the smallest atomic radius? A. Lithium B. Florine C. Bromine D. Chlorinearrow_forwardWrite the electron configurations for the elements below. 1.Magnesium 2.Calcium 3.Rubidiumarrow_forward
- Propsarrow_forwardDraw an “energy level diagram” for a calcium (Ca) atom. HINT: Use the periodic table to determine the number of electrons that are present in a krypton atom. An empty (no electrons) energy level diagram for multi-electron atoms is shown on the left. Electrons are arranged (configured) into the orbitals in the way that results in the lowest possible energy. Nature does this by obeying the Aufbau Principle, the Pauli Exclusion Principle, and Hund’s Rules. See the hint from the previous question (3.2) for more details. EXPLANATION: A calcium atom has 20 protons and therefore 20 electrons. Electrons are arranged (configured) into the orbitals in the way that results in the lowest possible energy. Nature does this by obeying the Aufbau Principle, the Pauli Exclusion Principle, and Hund’s Rules. See the hint from the previous question (3.2) for more details. Note that the 4s orbital fills before the 3d orbital because the 4s orbital lower in energy than the 3d orbitals (the 3d orbitals are…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY