
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please don't provide handwritten solution .....
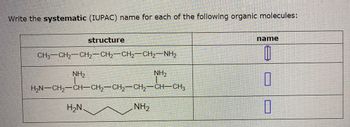
Transcribed Image Text:Write the systematic (IUPAC) name for each of the following organic molecules:
CH3CH,—CH,CH,CH2CH, NH,
NH₂
structure
H₂N
HẠN–CH,—CH–CH2–CH2–CH2CH—CH3
NH₂
NH₂
name
0
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5 What initial volume of a 24.00 M solution must have been present in order to allow for a 0.1000 Molar solution to have been created with a final volume of 1.800E6 mL of solution? There must have been an initial volume of type your answer... mLarrow_forwardPlease don't provide handwritten solution...arrow_forwardWhat volume (mL) of 0.2875 M NaOH is theoretically required to titrate 20.00 mL of 0.1276 M HCl? What is the percent error, if the volume of 0.2875 M NaOH that is experimentally required to titrate 20.00 mL of 0.1276 M HCl is found to be 11.84 mL?arrow_forward
- Please anwer question and exlpain whyarrow_forwardAt a particular temperature, suppose that 24.4 g solute is added to 50.0 g water and, after mixing, 0.3 g of the solute remains undissolved. What is the solubility of this solute at this temperature in grams per 100 g of water? (Enter in standard notation with one digit after the decimal point) Type your answer......arrow_forward1. Develop a detailed separation scheme for the separation and determination of the percent composition of your sample which will be a mixture of NaCl, NH.CL, and sand. You are expected to use the properties of the components listed below and some of the techniques listed in the table in the pre-lab queries (and used in Separations I). Keep in mind that any chemistry student should be able to pick up your scheme, understand it, and use it to complete the separation and calculation of % composition. Place the scheme on a separate sheet of paper. Component Solubility (@25°C) Melting Point Hardness sodium chloride, NaCl 35 g/100 mL water 801°C soft ammonium chloride, NH&Cl 37 g/100 mL water sublimes 350°C soft sand, SIQ2 insoluble 1600°C hard Example of compounds in a container. Naci NH.CI sio2 Exploring the Chemical World, PGCC, 2003 4 RESULTS Show all calculations with units in this space.arrow_forward
- Please don't provide handwritten solution ..... The average human body contains 5,830g of blood. What mass of arsenic is present in the body if the amount in blood is 0.55 ppm?arrow_forwardHow do I calculate/ what is the equation for the % weight/weight when I have a mass in grams and a volume of titrant delivered? Unknown Chrloride mass 0.1173g and 35.47 ml.arrow_forwardwww-awn.aleks.com O MEASUREMENT Deducing the unit missing from the solution to a basic qu.. A student sets up and solves the following equation to solve a problem in solution stoichiometry Fill in the missing part of the student's equation. 1 kg (135D 103 1 mL 0.65 kg (0.48 L) 3 10 ? 1 Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY