
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
3
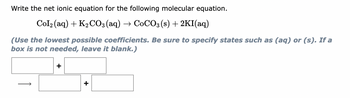
Transcribed Image Text:Write the net ionic equation for the following molecular equation.
Col₂ (aq) + K2₂CO3(aq) → CoCO3(s) + 2KI(aq)
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a
box is not needed, leave it blank.)
+
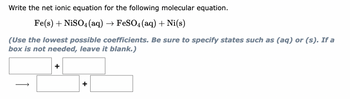
Transcribed Image Text:Write the net ionic equation for the following molecular equation.
Fe(s) + NiSO4 (aq) → FeSO4 (aq) + Ni(s)
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a
box is not needed, leave it blank.)
+
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- -Br 1.2 Li, Et₂O 2. C=CH 3. CH3(CH₂)2CIarrow_forwardCalculate the cost of one pound of nitrogen from a fertilizer containing 20.0% ammonium sulfate by weight and costing $8.10 per 100 lb. Express your answer in dollars per pound of nitrogen to three significant figures.arrow_forwardJO 1. CH 3 µg Br 2. H+arrow_forward
- Is this an Endothermic Sn1 diagram?arrow_forwardWrite the different methods of Equivalence Calculations?arrow_forwardHistory stry Bookmarks X O8 https://app.101edu.co FEB 13 Tools Window 80 Aktiv Chemistry F3 Balance the following chemical equation (if necessary): Si₂H3(s) + O₂(g) → SiO₂(g) + H₂O(g) 1 $ + Reset Help SIO₂ O F4 X 04 03- 3 0₂ 03 ) + F5 Question 40 of 47 0²- 4 0 ↑ H₂O < 0- 5 05 □+ ALL F6 6 0 1 MacBook Air 2 2+ & 7 Si₂H3 07 (s) (1) (g) (aq) 4 F7 8 9 0 08 09 Do • x H₂O 4+ ♫ A * 0₂ FB Delete tv w O O DD F9arrow_forward
- 4/3 pie * (0.0576/2)^3=0.00010061=1.00=1.00x2.20/60.02=0.036654449*60.09=2.202565811=2.2E-18 ? is this even right? i've been struggling with this problem for hours.arrow_forwardA chardonnay wine is 13.5 percent by mass alcohol. If we consume 24 fluid oz of wine, and the density of the wine is 0.982 mg/mL, how much alcohol was consumed? 09.6 x 103 g alcohol O 301 g alcohol O 94 g alcohol O 106 g alcohol O 13.3 g alcoholarrow_forwardTo aid in the prevention of tooth decay, it is recommended that drinking water contain 0.900 ppm fluoride, F". How many grams of F must be added to a cylindrical water reservoir having a diameter of 5.29 x 10° m and a depth of 65.43 m? mass: gF How many grams of sodiumn fluoride, NaF, contain this amount of fluoride? mass: 8 NaFarrow_forward
- A piece of platnium metal ore obtained was determined to have a mass of 1.6124 grams. The density of platnium is 21.45 g/ml what is the full volume (in liters) of the piece of platniumarrow_forwardthe Daily Value for saturated fat is 20 grams (g), which equals 100% DV. If the Nutrition Facts Label says one serving of a food contains 1.5 g of saturated fat, how much % DV this meals contains from the maximum amount of fat that is recommended for you per day.arrow_forwardThe legal limit for chromium in drinking water is 0.10 ppm. What is the maximum permissible mass of chromium (in \mu gμg) in exactly 1.0 cup (8.0 fl. oz.) of drinking water? Assume the density of drinking water is 1.00 g/mL. (1 fl. oz. = 29.57 mL)(Express your answer with two significant digits.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY