
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Write the name of each compound. Give explanation.
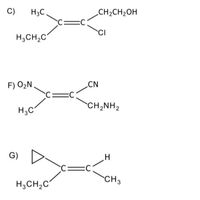
Transcribed Image Text:C)
H3C
CH2CH2OH
C=C
CI
H,CH,C
F) O2N
CN
C=
CH,NH2
H3C
G)
c=C
CH3
H3CH,C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1) Pretend there is a new metal wagium with the symbol Wg, and it is a variable charge metal. It makes the compound WgN. What is the name of this compound? What type of compound is this? What is the charge on the metal? 2) Pretend there is a new metal Purrium with the symbol Pi, and it is a fixed charge metal. It makes the compound Pi(SO4)2. What is the name of this compound? What type of compound is this? What is the charge on the metal? How are the nomenclature rules different for these two compounds?arrow_forwardGive only typing answer with explanation and conclusionarrow_forwardPart 4: Answer the following question. 12. Using the vocabulary terms atom and element appropriately, describe what makes up the compound CO2. Hint: Think back to the example given in the notes like this.arrow_forward
- For some questions you will need to use a special periodic table which is attached below Treat Je, Qu, Ap, and Bg as NONMETALS! a) Give the IUPAC name for the following: (look at attached images) b) Draw the condensed structural formula for 3-bogusohexanal.arrow_forwardPlease name the following compounds.arrow_forwardings ools Molecular compounds are usually composed solely of nonmetals. A binary molecular compound is one in which the compound contains only two elements (regardless of how many atoms are present of each). When naming binary molecular compounds, prefixes are used to specify the number of atoms of each element. Take a moment to review some of the prefixes shown here. Prefix Number mono di nona one three tetra four penta five hexa six hepta seven octa eight nine deca two ten For example, SF6 is named sulfur hexafluoride. Note that the prefix mono is not used in naming the first element. Also note that the second element in the name should end with the suffix ide. ▼ Part A Using the rules for naming molecular compounds described in the introduction, what is the name for the compound PC15? Spell out the full name of the compound. ►View Available Hint(s) Submit Part B Using the rules for naming molecular compounds described in the introduction, what is the name for the compound N₂ CL?…arrow_forward
- spell out the full name of the compoundarrow_forwardWhat is the name of the compound with the formula AII3? Name: What is the name of the compound with the formula NaCl ? Name: What is the name of the compound with the formula BaS ? Name: What is the name of the compound with the formula CaBr2 ? Name: What is the name of the compound with the formula K2O ? Name:arrow_forwardType the name of the compound that corresponds to the formula given in the following table. Enter the name in lowercase letters except for roman numerals. Compound Name Formula KMnO4 Co(NO3)3 SnCl2 NiSO4 AsF5 CrC204arrow_forward
- What is the name of the compound with the chemical formula KBr? Spelling counts. name: What is the name of the compound with the chemical formula K, Se? Spelling counts. name:arrow_forwardQUESTION 12 Give the chemical formula for each name. Do not worry about making the numbers subscripts, because this feature may not be available to you. Magnesium Hydroxide Tetraphosphorus Trisulfide Mercury (II) Sulfate Gold (III) Chloride Dinitrogen Pentoxide Ammonium Phosphatearrow_forwardenter the formula for each ionic compounds. express your answer as a chemical formula. Lead(II) chromate Iron (III) fluoride Iron(II) phosphate Potassium hydroxidearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY