
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
If possible to draw the mechanisms and products of the last row of compounds for all nu:
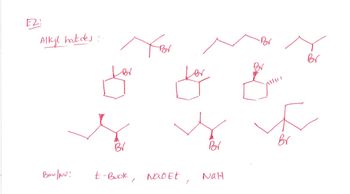
Transcribed Image Text:E2
Alkyl halides:
Br
Bor
Br
Br
Br
BV
Ban/NU:
t-Buok, Nao Et
NaH

Transcribed Image Text:Write the mechanism for all combinations of
reactions given in this handout (attached below).
Include stereochemistry when possible. Write and
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- If possible to draw mechanisms of each combo for just the first row of compoundsarrow_forward3c)Referring to the intermediates you drew in problem below explain in detail why no meta product is obtained in the Friedel-Crafts alkylation of chlorobenzene. Draw all pertinent resonance structures to support your argument.arrow_forwardGive detailed mechanism Solution with explanation needed..don't give Handwritten answerarrow_forward
- Give detailed mechanism Solution with explanation needed..don't give Handwritten answer..give also type of reaction..SN1 ,SN2, E1 and E2arrow_forwardd) In any Friedel-Crafts alkylation reaction starting with benzene and using one equivalent of the alkylating reagent, there is a always a small amount of disubstituted product which is formed. By contrast, such outcomes are not typically observed for any halogenation reactions starting from benzene and similarly using EAS chemistry. Please explain the basis for this apparent dichotomy.arrow_forward(b) Discuss about the stability factors of the reaction intermediates, which involved in a name reaction "Wittig rearrangement".arrow_forward
- 3. For the following variants of the rearrangement reactions we have covered in class, propose a feasible step-by-step mechanism for each of them. (a) NaOMe MeOHarrow_forwardGive detailed Solution with explanation needed..please explain....for a good reviewarrow_forwardGive detailed mechanism Solution with explanation needed...don't give Handwritten answer..complete the following reactionsarrow_forward
- Give detailed Solution with explanation needed...predict the reactant/ product..give the mechanism....arrow_forwardGive detailed mechanism Solution with explanation needed...don't give Handwritten answerarrow_forward[References] Draw the expected major organic product of the Sharpless epoxidation of each allylic alcohol using (-)-diethyl tartrate as the chiral catalyst. (a) (b) All hydrogen atoms are implied. If a chiral atom is attached to a hydrogen atom, you should not show the hydrogen atom but use either a wedge or a dashed bond. Apply formal charges where appropriate. Omit lone pairs and radical electrons from your answer. • Omit + signs between structures. ● HO O HO -OCH3 کر ? ChemDoodle Previous Nextarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning