
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Write the equilibrium constant expression, Kc, for the following reaction:
| If either the numerator or denominator is 1, please enter 1 |
CO (g) + Cl2 (g) COCl2 (g)
| Kc = | |
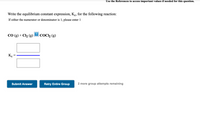
Transcribed Image Text:Use the References to access important values if needed for this question.
Write the equilibrium constant expression, Ke, for the following reaction:
If either the numerator or denominator is 1, please enter 1
CO (g) + Cl2 (g)
?
COC12 (g)
Ke
Submit Answer
Retry Entire Group
2 more group attempts remaining
Expert Solution
arrow_forward
Step 1
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student ran the following reaction in the laboratory at 538 K:COCl2(g) CO(g) + Cl2(g)When she introduced 1.24 moles of COCl2(g) into a 1.00 liter container, she found the equilibrium concentration of Cl2(g) to be 3.76×10-2 M.Calculate the equilibrium constant, Kc, she obtained for this reaction.Kc =arrow_forwardA student ran the following reaction in the laboratory at 290 K:2CH2Cl2(g) CH4(g) + CCl4(g) When she introduced 8.37×10-2 moles of CH2Cl2(g) into a 1.00 liter container, she found the equilibrium concentration of CH2Cl2(g) to be 6.14×10-3M. Calculate the equilibrium constant, Kc, she obtained for this reaction. Kc =arrow_forwardIn the context of equilibrium constants of chemical reactions, which "K" value indicates a reaction that favors the formation of products the most? K- 1.7 x 10-6 K= 8.2 x 10-3 OK- 5.31 x 10 OK- 4.99 x 106arrow_forward
- Consider the following equilibria A(g) → 2 X(g) + C(g) Kc = 1.55 B(g) → X(g) Kc = 25.2 Calculate the equilibrium constant for the following reaction? A(g) 2 B(g) + C(g) Kc = ? 6.10 x 10-4 26.8 2.44 x 10-3 78.1 984 Sarrow_forwardWrite the equilibrium-constant expression for the reaction A(s) + 3 B(1) ⇒ 2 C(aq) + D(aq) in terms of [A], [B], [C], and [D], as needed. Note that Kc, which is sometimes symbolized as Keq, denotes that the equilibrium constant is expressed using molar concentrations. For this question, Kc means the same thing as Keq. Kc =arrow_forwardWrite the equilibrium constant expression, K , for the following reaction: (If either the numerator or denominator is blank, please enter 1.) K=arrow_forward
- Write the equilibrium constant expression, K, for the following reaction: If either the numerator or denominator is blank, please enter 1 Ag2SO3(s) =2Ag+(aq) + SO3²-(aq) K =- %3Darrow_forwardWrite the equilibrium constant expression, Kc, for the equation: 2NO2(g) + 7H2(g) -----> 2NH3(g) + 4H2O(g) Write the equilibrium constant expression, Kc, for the reverse of the previous reaction.arrow_forwardWrite the equilibrium constant expression, K, for the following reaction: If either the numerator or denominator is 1, please enter 1 CH4(g) + CCl4(g) 2CH2Cl2(g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY