
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
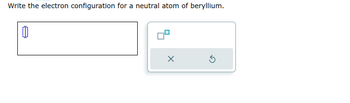
Transcribed Image Text:Write the electron configuration for a neutral atom of beryllium.
0
x
5
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- cmillan Learm Write the full electron configuration for P³-. full electron configuration: 1s22s22p63s23p6 What is the atomic symbol for the noble gas that also has this electron configuration? atomic symbol:arrow_forwardGive the electron configurations for the following atoms. Do not use the noble gas notation. Write out the complete electron configuration. Example: Mg 1s2s²2p°3s² Element Electron configuration N Sc Li Cuarrow_forwardIn general, the ionization energy increases as you move from left to right in the periodic table, as can be seen in the figure below. 2500 He Noble gases Ne 2000 Ar * 1500 Kr Period 4 transition elements Period 5 Хе transition elements 1000 500 Li Na K Rb Alkali metals 0+ 10 20 30 40 50 Atomic number However, the ionization energy of boron lower than that of beryllium, and the ionization energy of aluminum is lower than that f magnesium.arrow_forward
- Photoelectron spectroscopy applies the principle of the photoelectric effect to study orbital energies of atoms and molecules. High-energy radiation (usually UV or X-ray) is absorbed by a sample and an electron is ejected. The orbital energy can be calculated from the known energy of the radiation and the measured energy of the electron lost. The following energy differences were determined for several electron transitions: ΔE2 →1 = 4.098 ×10−17 J ΔE3 →1 = 4.854 × 10−17 JΔE5 → 1 = 5.242 ×10−17 J ΔE4 → 2 = 1.024 ×10−17 J Calculate the energy change and the wavelength of a photon emitted in the following transitions. Enter your answers in scientific notation. Use 6.626 ×10−34 J·s for Planck's constant. (a) Level 3 to 2: ______J ______m (b) Level 4 to 1: _____J_____ m (c) Level 5 to 4: _____J _____marrow_forwardWrite down the orbital diagram for an ion that contains 19 protons and 18 electrons.arrow_forwardReorder the list in the table, if necessary, so that the atoms and ions in it are list in order of decreasing size.arrow_forward
- An atom has the following electron configuration. How many valence electrons does the atom have? 1s²22s22p63s23p3arrow_forwardWhich elements have only one electron shell? carbon hydrogen heliumarrow_forward(a) Identify the number of electrons in the ground-state outer shell of atomic oxygen (atomic number 8).(b) How many electrons are in the ground-state outer shell of fluorine?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY