
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
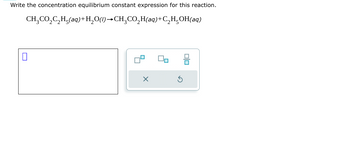
Transcribed Image Text:Write the concentration equilibrium constant expression for this reaction.
CH3CO2C2H5(aq)+H2O(l)→CH¸CO₂H(aq)+C2H5OH(aq)
☐
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Ionic Solids do not dissolve in non-polar solvents. (TRUE OR FALSE) (b) H3AsO4 is a weak acid. (TRUE OR FALSE) (c) Once a chemical reaction has come to equilibrium, the reaction comes to a complete halt.(TRUE OR FALSE)arrow_forwardIf you begin with 28.4 mL of 0.287 M HCl(aq) solution and mix it with 17.3 mL of 0.181 M NaOH(aq), what is the final pH when they are mixed together?HCl(aq)+ NaOH(aq)→ H2O(l) + NaCl(aq)arrow_forwardPhosphoric acid, H3PO4(aq), is a triprotic acid, meaning that one molecule of the acid has three acidic protons. Estimate the concentrations of all species in a 0.100 M phosphoric acid solution. pKa1 = 2.16 pKa2 = 7.21 pKa3 = 12.32 [H3PO4]= ? [H2PO4 -]= ? [HPO4 2-]= ? [PO4 3-] =? [H+] = ? [OH-]= ?arrow_forward
- Complete the balanced chemical reaction for the following weak base with a strong acid. In this case, write the resulting acid and base as its own species in the reaction. NH3(aq) + HCl(aq) – ->arrow_forwardBe sure to answer all parts. Determine Angas for each of the following reactions: (a) MgCO3(s) →→→ MgO(s) + CO₂(g) mol (b) 2 H₂(g) + O₂(g) → 2 H₂O() mol (c) HNO3(1) + CIF(g) → CIONO₂(g) + HF(g) molarrow_forwardWrite a balanced net-ionic equation for the neutralization reaction between CH3COOH (aq) and Ba(OH)2 (aq). 2 CH3COOH (aq) + Ba(OH)2 (aq) --> 2 CH3COO- (aq) + 2 H₂O (1) + Ba²+ (aq) 2 CH3COOH (aq) + 2 OH" (aq) --> 2 CH3COO(aq) + 2 H₂O (1) CH3COOH (aq) + OH" (aq) --> CH3COO (aq) + H₂0 (1) OH(aq) + OH- (aq) --> H₂O (1) 2 H (aq) + 2 OH- (aq) --> 2 H₂O (1)arrow_forward
- true or false? The following reaction equation is a redox reaction: 2H2O (l) + 2Cl- (aq) ⟶ H2 (g) + Cl2 (g) + 2OH- (aq)arrow_forwardQ) Drinking water may contain several unwanted ions such as phosphate ions. In order to remove the phosphate ions from the drinking water, a solution of calcium hydroxide can be added. As a result, a solid Ca5OH(PO4)3 is formed and isolated by filtration and a hydroxide ion are also formed. The reaction for this process is below: 5 Ca(OH)2 (aq) + PO43- (aq) → Ca5OH(PO4)3 (s) + OH- (aq) a) If 3.00 mL of 0.100 M calcium hydroxide is mixed with 4.00 mL of an aqueous solution of 0.0800 M phosphate ions (PO43-). What is the mass of Ca5OH(PO4)3 that can be isolated from the reaction? b) How many moles of the excess reactant remains unreacted? c) What is the concentration of the hydroxide ions in this solution? d) What is the mass of calcium (in grams) that can be recovered?arrow_forwardCalculate the analytical molar concentrations of trichloroacetic acid in an aqueous solution that contains 152.9 mg of trichloroacetic acid,Cl3CCOOH (163.4 g/mol), in 19.3 mL (the acid is 73% ionized in water). Indicate unitsarrow_forward
- The maximum contaminant level of cyanide (CN) in drinking water as set by the the Environmental Protection Agency (EPA) is 0.00020 g · L. Express this concentration in parts per million (ppm). Assume the density of water is 1.00 g/mL. concentration: Ppmarrow_forwardWrite a balanced net ionic equation for each of the following acid–base reactions. HCl(aq)+KOH(aq)→KCl(aq)+H2O(l)HCl(aq)+KOH(aq)→KCl(aq)+H2O(l) Express your answer as a net ionic equation. Identify all of the phases in your answer.arrow_forwardA titration involves adding a reactant of known quantity to a solution of an another reactant while monitoring the equilibrium concentrations. This allows one to determine the concentration of the second reactant. The equation for the reaction of a generic weak acid HA with a strong base is HA(aq) + OH(aq) →A¯(aq) + H₂O (1) A certain weak acid, HA, with a K, value of 5.61 x 10 ", is titrated with NaOH. 6 Part A A solution is made by titrating 9.00 mmol (millimoles) of HA and 2.00 mmol of the strong base. What is the resulting pH? Express the pH numerically to two decimal places. ▸ View Available Hint(s) pH = Submit ▾ Part B pH = IVE] ΑΣΦ Submit A → c More strong base is added until the equivalence point is reached. What is the pH of this solution at the equivalence point if the total volume is 66.0 mL ? Express the pH numerically to two decimal places. ▸ View Available Hint(s) IVE ΑΣΦ A + Ċ ? Doowoon Review | Constants | Periodic Table ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY