
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
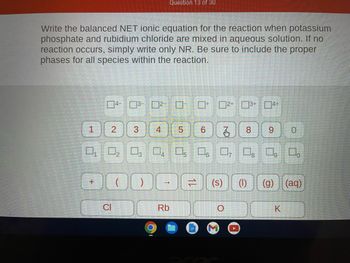
Transcribed Image Text:Write the balanced NET ionic equation for the reaction when potassium
phosphate and rubidium chloride are mixed in aqueous solution. If no
reaction occurs, simply write only NR. Be sure to include the proper
phases for all species within the reaction.
1
+
4-3-²--
2
J
0₁ 0₂ 03 04 05 06 0, 0, 0, 0.
DOO
CI
3
Question 13 of 30
4
-
Rb
À
5 6
12
2+ 3+ 4+
=
8 9 0
(s) (1) (g) (aq)
K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the balanced COMPLETE ionic equation for the reaction when KCl and Na₃PO₄ are mixed in aqueous solution. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction.arrow_forward[References) Use the References to access important values if needed for this question. In the laboratory you are given the task of separating Ag* and Fe2+ ions in aqueous solution. For each reagent listed below indicate if it can be used to separate the ions. Type "Y" for yes or "N" for no. If the reagent CAN be used to separate the ions, give the formula the precipitate. If it cannot, type "No" Y or N Reagent Formula of Precipitate if YES 1. K,CO, 2. NaCl 3. Na,8O, Submit Answer Try Another Version 3 Item attempts remaining (Previous Next) 10 stv MacBook Air DII 80 888 F4 F7 F8 F9 F3 F5 F6 %23 $ % & 8 %3D 3 4 { E Y G H L C V M command option .. .-arrow_forwardWrite the full balanced equation with state signs for: An aqueous solution of Iron (II) Chloride is mixed with aqueous Sodium Phosphate forming solid Iron (II) Phosphate and Sodium Chloride.arrow_forward
- Write the balanced dissociation equation for solid iron(III) nitrate in aqueous solution. If it does not dissociate, simply write only NR. Be sure to include the proper phases for all species within the reaction.arrow_forwardWrite the balanced molecular equation for the reaction between silver nitrate and the iodide ion.arrow_forwardConsider the chemical reaction that takes place between aqueous hydrochloric acid and aqueous sodium hydroxide. Write the balanced NET ionic equation for the reaction. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction.arrow_forward
- Suppose you are asked to determine the identity of a white solid. When you add water to the white solid, it dissolves and forms a clear solution. When you add a solution of Na, SO,, a white precipitate forms. Add Add H,O Na,SO, Determine the identity of the original solid. O PBCI, O MgCl, O ZNCI, O CaCl,arrow_forwardWrite the balanced net ionic equation for the reaction. Include phases. (The video shows the addition of aqueous sodium carbonate to a solution of aqueous magnesium nitrate.)arrow_forward4. Using the solublity chart we can see that compound is soluble KNO3 PBCI Ca(OH)2 Aglarrow_forward
- A group of students combined HCl (aq) and Al (s). The student observed bubbling, thus a reaction occurred. Write the balanced molecular equation for this reaction showing coefficients and phase labels. Write the complete ionic equation for this reaction. Write the net ionic equation for this reaction.arrow_forwardA student adds silver nitrate solution to hydrochloric acid solution and obtains a white precipitate. The student then adds a clear, colorless solution of an unknown metal ion to a solution of hydrochloric acid, and the result is clear and colorless. What can the student conclude about the unknown?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY