
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
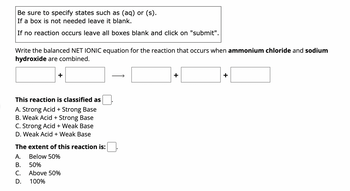
Transcribed Image Text:Be sure to specify states such as (aq) or (s).
If a box is not needed leave it blank.
If no reaction occurs leave all boxes blank and click on "submit".
Write the balanced NET IONIC equation for the reaction that occurs when ammonium chloride and sodium
hydroxide are combined.
This reaction is classified as
A. Strong Acid + Strong Base
B. Weak Acid + Strong Base
C. Strong Acid + Weak Base
D. Weak Acid + Weak Base
The extent of this reaction is:
A.
Below 50%
B.
50%
C.
Above 50%
D.
100%
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the reaction HC1O+F HF + CIO¯ Which of the following statements is true: A. It is product favored and acidic O B. It is reactant favored and acidic C. This makes a neutral solution O D. It is reactant favored and basic E. It is product favored and basic Reset Selectionarrow_forwardUESTION 7 Determine the hydronium ion concentration for solutions with the following pH values: a. pH = 9.3 b. pH = 2.96 c. pH = 7.02 %D d. pH = 8.095arrow_forward8) What is the most accurate way to see acidity or alkalinity? a. blue litmus paper b. red litmus paper c. universal litmus paperarrow_forward
- Q 11 pleasearrow_forwardWrite the balanced NET IONIC equation for the reaction that occurs when hydroiodic acid and ammonia are combined. Use H3O+ instead of H+. + + This reaction is classified asfill in the blank 5.A. Strong Acid + Strong BaseB. Weak Acid + Strong BaseC. Strong Acid + Weak BaseD. Weak Acid + Weak BaseThe extent of this reaction is:fill in the blank 6.A. ... Below 50%B. ... 50%C. ... Above 50%D. ... 100%arrow_forwardQuestion is attached.arrow_forward
- Need answers to h-karrow_forward82. The hydrogen sulfide ion, HS , is a weak base. a. Write the equilibrium reaction between HS¯ and water. Label the main conjugate acid-base pair. b. Is HS a strong or weak electrolyte? c. If additional hydroxide ions, OH , are added to the solution, will the reaction shift to the left or to the right? Explain. d. If some HS ions are removed from solution, will the reaction shift to the left or to the right? Explain.arrow_forwardPart 1. Evaluate each statement and determine whether it is true or false Part 2. Classify the compounds as Arrhenius acid or Arrhenius base. Write N/A if it does not fit an of the classifications Part 3. Predict the products of the reaction of each pair of Arrhenius acid and Arrhenius base and write it's balanced equationarrow_forward
- In the reaction NH3 + H2O → NH4+ + OH–, the Bronsted-Lowry base is __________ a. NH4+ b. NH3 c. OH– d. H2Oarrow_forwardWhich of the following statements is incorrect? A. A neutral solution contains equal amounts of hydronium and hydroxide ion B. An acidic solution contains low concentration of hydronium ions C. A acidic solution contains high concentration of hydronium ion D. A basic solution contains high concentration of hydroxide ionsarrow_forwardThe pH of a solution of HClO4 was found to be 3.4. The concentration of this solution in mol/L is which of the following? A. B. C. 4.0 × 10-49 3.4 0.29 D. E. 2.5 × 10-11 15. none of the abovearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY