
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How can we solve the solutions at 25 degrees Celsius? And how can we write the balanced equation?
Transcribed Image Text:Macmillan Learning
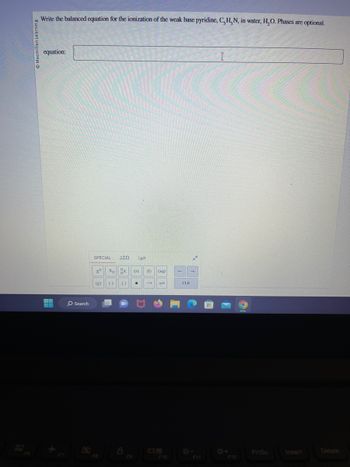
Write the balanced equation for the ionization of the weak base pyridine, C, H, N, in water, H₂O. Phases are optional.
equation:
K
Search
SPECIAL ΔΣΩ 2μm
xº
(g)
Xp X (S)
() 11 ●
8
F9
(1) (aq)
→
F10
←
CLR
→
I
F12
PrtSc
Insert
Delete
![k
Ⓒ Macmillan Learning
Calculate either [H₂O+] or [OH-] for each of the solutions at 25 °C.
Solution A: [OH-] = 1.37 x 10-7 M; [H₂O+] =
H
Solution B: [H₂O+]=9.51 x 10-9 M; [OH-] =
Solution C: [H₂O+] = 7.55 x 10-4M; [OH-] =
Which of these solutions are basic at 25 °C?
Solution A: [OH-] = 1.37 x 10-7 M
Solution B: [H₂O+] = 9.51 x 10° M
Solution C: [H3O+] = 7.55 x 10-4 M
O Search
F10
+F12
PrtSc
Resources Ly
M
M
M](https://content.bartleby.com/qna-images/question/941b2278-47bd-4d45-a54e-f66e352d4961/cca69531-ea19-4ab2-be51-ced724514125/2yyno8y_thumbnail.jpeg)
Transcribed Image Text:k
Ⓒ Macmillan Learning
Calculate either [H₂O+] or [OH-] for each of the solutions at 25 °C.
Solution A: [OH-] = 1.37 x 10-7 M; [H₂O+] =
H
Solution B: [H₂O+]=9.51 x 10-9 M; [OH-] =
Solution C: [H₂O+] = 7.55 x 10-4M; [OH-] =
Which of these solutions are basic at 25 °C?
Solution A: [OH-] = 1.37 x 10-7 M
Solution B: [H₂O+] = 9.51 x 10° M
Solution C: [H3O+] = 7.55 x 10-4 M
O Search
F10
+F12
PrtSc
Resources Ly
M
M
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H2O).arrow_forward50.0g of NaCl was added to 500.0g of water. Calculate the mass percent of NaClarrow_forwardHow many moles of potassium bicarbonate, KHCO3, are present in 75.0 mL of a 0.750 M potassium bicarbonate solution?arrow_forward
- The solubility of CuBr in water at 25 °C is measured to be 0.010 L Round your answer to 2 significant digits. 0 Dx1 x10 Ś Use this information to calculate K for CuBr. sparrow_forwardlons move freely in solution. O True O Falsearrow_forwardDetermine the concentration of a solution prepared from 10.5 kg of magnesium sulfate dissolved in 13.7 L of waterarrow_forward
- The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). Note: "major" chemical species are those present in concentrations greater than 10 -9- mol/L. dlo major species present when dissolved in water compound formula glycerol C,H,O, isopropanol C,H,0 nickel(II) chloride NiCl,arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). -6 Note: "major" chemical species are those present in concentrations greater than 10 mol/L. major species present when dissolved in water compound formula 0,0,.. nickel(II) chloride NiCl, sodium nitrate NaNO3 nickel(II) iodide Nil,arrow_forwardThe names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H₂O). Note: "major" chemical species are those present in concentrations greater than 10 mol/L. compound methanol fructose iron(II) iodide formula CH₂OH C6H12O6 FeL₂ major species present when dissolved in water 0 X 0,0,... Śarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY