
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Chemi X o Mail - Thomps
O8 https://app.101edu.co
F2
#
P Parchment Red TW6D2PHI.pdf
3
80
F3
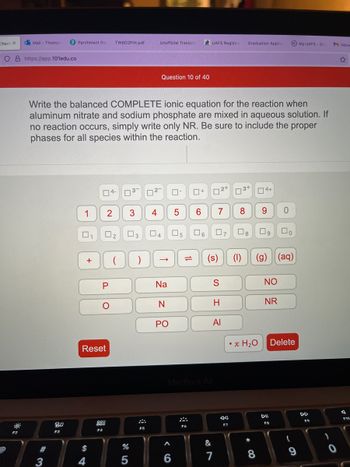
Write the balanced COMPLETE ionic equation for the reaction when
aluminum nitrate and sodium phosphate are mixed in aqueous solution. If
no reaction occurs, simply write only NR. Be sure to include the proper
phases for all species within the reaction.
1
0
1
+
$
4
04-
Reset
F4
2
0₂
P
(
%
5
³- 2
3
F5
Unofficial Transcri
4
Question 10 of 40
→
Na
N
PO
A
0
6
5
05
0+
F6
UAFS Registra Graduation Applic
6
6 7
MacBook Air
(s)
&
72+
S
H
Al
7
7
F7
3+
8 9 0
08
(1) (g)
• x H₂O
4+
*
8
NO
NR
FB
口。
(aq)
Delete
My.UAFS-Stu
9
DD
M Inbox
0
F10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 1.00 gram sample of anhydrous cerium sulfate was dissolved in water. Barium nitrate solution was then added until no further precipitation occurred. The precipitate was filtered from the solution then dried and was found to weigh 1.41 g. Determine the formula of the cerium sulfate. Encom calt is hydratedarrow_forwardWrite the balanced COMPLETE ionic equation for the reaction when iron(III) chloride and sodium sulfide are mixed in aqueous solution. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction.arrow_forwardUsing the solubility rules, predict the formula of the precipitate, and write a balanced equation describing the reaction.arrow_forward
- Calculate the molarity of 150.0 mL of sodium hydroxide solution needed to react with 65.7 mL of a 0.128 M solution of phosphoric acid. OA. 0.877 M Ов.0.168 м Oc. 0.0974 M OD. 0.0420 M OE. 0.0187 M OF. 0.0390 M OG. 0.0561 M OH.0.292 M QUESTION 2 What volume of 0.128 M solution of hydrochloric acid is needed to react with 200.0 mL of 0.128 M calcium hydroxide? O A. 0.0000410 mL Ов. 100. mL OC. 0.000164 mL OD. 24400 mL OE. 400. mL OF. 0.0000819 ml OG. 200. ml OH.6100 mL QUESTION 3 Given a density of 1.17 g/mL, what volume of a 50.5% solution of sulfuric acid is needed to react 350.0 mL of 0.138 M strontium hydroxide? OA. 95.6 mL Ов. 13100 mL OC. 5.15 mL OD.6.02 mL OE. 9.38 mL OF. 8.02 mL OG. 15300 mL OH.4.40 mL QUESTION 4 Given 200.0 mL of a 0.139 M solution of aluminum chloride, according to the following equation, how many mL of a 0.358 M solution of sodium carbonate are needed to react with the aluminum chloride? 3 NazcOg(aq) + 2 AICI3(aq) - Alg(coz)a(s) + 6 Naci(aq) OA. 77.7 ml OB.…arrow_forwardWrite the (i) balanced conventional equation (CE), (ii) total ionic equation (TIE), and (iii) net ionic equation (NIE) for each of the reactions below. Include all state symbols. Assume that, if there is a reaction, it will go to completion. If there is no reaction, write "No Reaction" for the NIE but still complete the CE and TIE. a. Aqueous solutions of potassium sulfate and calcium iodidearrow_forwardWrite the overall equation, total ionic equation, and net ionic equation for the reaction of phosphoric acid with potassium hydroxide.arrow_forward
- 2. Calculate the mass of the precipitate formed when 2.00 g of Barium Nitrate is mixed with an excess of Sodium Phosphatein water. Write out the balanced Molecular Equation .arrow_forwardA group of students combined HCl (aq) and Al (s). The student observed bubbling, thus a reaction occurred. Write the balanced molecular equation for this reaction showing coefficients and phase labels. Write the complete ionic equation for this reaction. Write the net ionic equation for this reaction.arrow_forward1. Aqueous copper (II) sulfate and 0.75 M sodium hydroxide react to form a precipitate of copper (II) hydroxide and aqueous sodium sulfate. Write the chemical equation for this reaction. Classify this reaction and write the general form for this type of reaction equation If both solutions were clear add the beginning of the reaction, why does this reaction form a blue solid precipitate? 2. Solid zinc metal reacts with aqueous hydrogen monochloride to form aqueous zinc chloride and hydrogen gas. Write the symbolic form of this chemical reaction. Classify this chemical reaction and write the general form of this type of reaction. What could we do to verify that the gas released was, in fact, hydrogen? Is zinc chloride soluble or insoluble in water? How do you know?arrow_forward
- What is the best way to visualize the ions in a chemical reaction? • The best way to visualize the ions is by studying them with an electron microscope • The best way to visualize the ions is by writing and balancing a chemical reaction • The best way to visualize that ions is by drawing a representation of them •arrow_forward1) Lead (I) nitrate reacts with sodium chloride. A) Write a balanced chemical equation to describe this reaction B) 100.0 mL of a lead (I) nitrate solution reacts with excess sodium chloride solution and produces 6.95 grams of precipitate. 2) Calculate the moles of the precipitate produced by the reaction. A) Calculate the moles of lead in the original solution. B) Calculate the molarity of the 100.0 mL lead (I) nitrate solution.arrow_forwardWrite the balanced equation for the reaction between hydrochloric acid solution and sodium hydroxide solution.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY