
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please help!
Transcribed Image Text:du.co
@
2
outlook.office365.com
#3
X
C
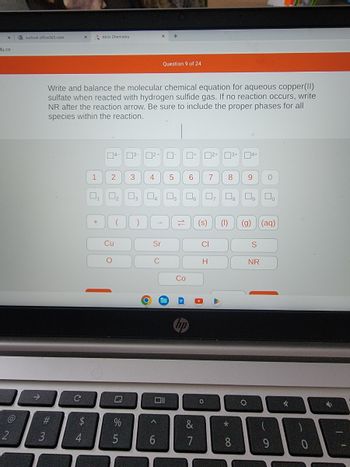
Write and balance the molecular chemical equation for aqueous copper(II)
sulfate when reacted with hydrogen sulfide gas. If no reaction occurs, write
NR after the reaction arrow. Be sure to include the proper phases for all
species within the reaction.
S4
1
Aktiv Chemistry
+
04-0³-
2
Cu
O
%
3
( )
5
f
0²-0-
O
4
Sr
Question 9 of 24
C
<6
9
O
5
05
14
Co
6
Ď
&
7
(s) (1)
CI
H
2+ 3+ 4+
7
O
8 9 0
*
¹8
8
口。 00
(g) (aq)
O
S
NR
-
S
✓
10
I'
Expert Solution
arrow_forward
Step 1
Balanced chemical equation:
Balanced chemical equation can be define as the reaction in which number of atoms of all the elements on the reactant side are equal to the number of atoms of all the elements on the product side.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Construct the expression for Kc for the following reaction. 1 3NO(g) = N20(g) + NO2(g) Construct the expression for Kc for the following reaction. 2 X 4HCI(aq) + O2(g) = 2H20(1) + 2C12(g) > Construct the expression for Kc for the following reaction. 3 CH4(g) + 2H2S(g) = CS2(g) + 4H2(g) Construct the expression for Kc for the following reaction. 4 X 2Cu*(aq) + Zn(s) = 2Cu(s) + Zn²*(aq) >arrow_forwardA 2.5 L canister under a pressure of 150 kPa at 20 °C is compressed to a volume of 1.5 L at 35 °C. What is the resulting pressure? K H Li Be Na Mg "K Ca Sc Ti V Cr Rb Cs Fr Ba Ra La La 158.2 kPa 329.3 kPa 56.8 kPa 180.3 kPa 98.3 kPa 262.8 kPa M 89-108 104 M Hf Ta Ac Metal Rf Db Sg Periodic Table of the Elements W Re Os A He Aline TH Symbel 41 42 M45 Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Basic 13 IKA LAS B Pt MAN 14 VE 15 AI Si P 17 VILA Cl w! Mn Fe Co NI Cu Zn "Ga Ge As Se Br Kr Neble LIONS Au Hg Tl Pb Bi Po At Rn 108 10 Sg Bh Bh Hs Mt Ds Rg Cn Uut "FI Uup "Lv Uus Uuo VIA He Ne 18 Yb Lu Pa U Np Pu Am Cm Bk Cf Es Fm Md No Ararrow_forwardWhat is the molarity of C₁2H22O11 in an aqueous solution that is prepared by dissolving a 29.87 g sample of impure C12H22011 in water to make 725.0 mL of solution. Assume that the sample is 83.0% C12H22011 by mass. Enter your answer in mol L-1, accurate to three significant figures. Do not include units as part of your answer. Use the following molar masses, in g mol-¹, for your calculations. H, 1.008 Number C, 12.01 0, 16.00arrow_forward
- help mearrow_forwardExplain/define and give examples for each chemistry terms: Digestion Gathering Particle growth Gravimetric analysis Peptization Precipitation Precipitantarrow_forwardPost-Laboratory Questions 1) An extra strength antacid tablet contains 750 mg of active ingredient, CaCC 22.25 mL of HC1 (aq) to neutralize the tablet, how strong is the acid? 2 HC1 (aq) + CaCO3 (aq) → CaCl,(aq) + HOH (1) + CO2 (arrow_forward
- Which of the following methods is not an appropriate method for purifying a solid under normal laboratory conditions? sublimation Ochromatography distillation recrystallizationarrow_forwardBalance each of the following neutralization reactions. Part A HNO, (aq) + Sr(OH)2(s)→H20(1) + Sr(NOs)2(aq) Express your answer as a chemical equation. Identify all of the phases In your answer. ΑΣφ ? DA chemical reaction does not occur for this question. Submit Request Answer t Speec.pdfarrow_forwardcalculate the mass and volume (mL) of 5.0 mmol N,N-diethylethanolamine mw N,N-diethylethanolamine: 117.9g/mol density: 0.883 g/mLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY