
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please write clearly the answer, as well as the type of compound it is. Such as gas, solid, etc
Transcribed Image Text:Question 32 of 37
Submit
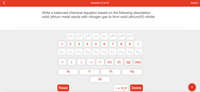
Write a balanced chemical equation based on the following description:
solid yttrium metal reacts with nitrogen gas to form solid yttrium(III) nitride
| 4-
2+
3+
4+
1
2
3
7
8
O2
O5
(s)
(1)
(g) (aq)
+
Y
Yt
Yb
Ni
Reset
• x H,O
Delete
+
CO
2.
4.
3.
3.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Write formula for cobalt(II) nitride Copper(III) carbonate. iron(I) sulfate.arrow_forwardClassify each of the following compounds as ionic (I), molecular (M), binary acid (BA), or oxyacid (OA). Then, name each compound.arrow_forwardwhat is the difference beteween ionic and covalent in simple terms? I have confused myself. What is a specific chemical reaction that is an example of each?arrow_forward
- Which name best describes a compound consisting of magnesium (a group II, main group metal) and oxygen (a group VI, main group non-metal). -- Hint: Is this an ionic compound or a molecular compound? Magnesium dioxide Magnesium (II) oxide This compound probably does not exist. Magnesium monoxide None of the answers are correct.arrow_forwardFormula of Calcium bicarbonate? ( including the positive ion and negative ion).arrow_forward19 10 15 6 7 18 11 12 13 14 15 16 17 Name brood Puzzled by the Concepts: 5 Naming 1 More Time Revised summer 2019-2022 S. Drake It is IMPORTANT to decide what kind of compound you have in order to name it. So, for the following compounds decide the type (simple ionic, transition metal ionic, polyatomic ionic, transition metal ionic & polyatomic ionic, or covalent) and then write its name or formula. Formula of compound 1 2 3 4 TIU2 Type of compound no (1) 1990 (V) muibansv hind aunorigaoriq byd munimuls ua (III) lexbin P Na₂CO3 P₂O5 CoBr2 FeSO4 SiO₂ CO SI CuCl2 SO₂ FeS NaBr Ca(C₂H302)2 Ti(SO4)2 FePO4 K3N CuOH Zn(NO₂)2 V₂S3 Tanel P CHE 106-17 Name of compound DO NOT capitalize. Disodium Carbonate T14arrow_forward
- Explain how to name ionic compound containing type II metals. Give two examplesarrow_forward7. Determine the type of compound (molecular, ionic, or acid) described below. Some may have more than one answer. See the table in the Introduction. a) Has high melting and boiling points b) Conducts electricity in the aqueous state pentachiorid c) Composed of metallic and nonmetallic atomsarrow_forwardGive an example of a binary diatomic formula unit, triatomic molecule of a compound, and triatomic molecule of an element.arrow_forward
- Provide the correct formula for sulfur tetrachloridearrow_forwardOrder them in decreasing order of the amount of oxygen. = Chlorate = Hypochlorite = Chlorite = Perchloratearrow_forwardDrag the compound names from the list and drop them into the appropriate buckets for ionic or molecular compounds. Provide your answer below: Give detailed Solution with explanation needed. don't give Handwritten answer Ionic Molecular sodium peroxide rubidium phosphate sulfur trioxide carbon dioxide potassium carbonate sodium sulfide lithium sulfate hydrogen iodide potassium hydride diphosphorous pentoxide hydrogen sulfide hydrogen peroxide potassium iodidearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY