
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Write
1,2,3-trimethylcyclohexane
a conformational structure for
in which all the
methyl groups are axial and then show its more stable
conformation.
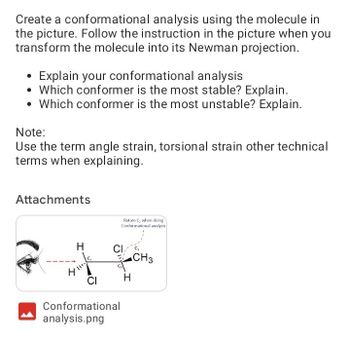
Transcribed Image Text:Create a conformational analysis using the molecule in
the picture. Follow the instruction in the picture when you
transform the molecule into its Newman projection.
Explain your conformational analysis
Which conformer is the most stable? Explain.
• Which conformer is the most unstable? Explain.
Note:
Use the term angle strain, torsional strain other technical
terms when explaining.
Attachments
H
4 111
CI
Rotate C, when doing
Conformational analysis
CI
Conformational
analysis.png
H
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q2. Answer any TWO of the following parts: (a) Draw the two main conformations that exist for cyclohexane. Explain clearly why one conformer is more stable than the other. Using cis-1-ethyl-3-methylcyclohexane, as an example, explain how ring flipping occurs. Draw both conformers of cis-1-ethyl-3-methylcyclohexane and explain clearly which one predominates. (b) What is polarimetry? The specific rotation of (R)-carvone is - 61°. A chemist prepared a 750 mg mixture of (R)-carvone and its enantiomer in 10 ml of ethanol and placed the solution in a 10 cm polarimeter cell. The observed rotation was - 4.125°. Calculate the specific rotation for the above mixture. What is meant by enantiomeric excess? Then determine the % enantiomeric excess (% ee) in the mixture. (i) (ii) (iii) What % of the mixture is (R)-carvone and (S)-carvone?arrow_forwardFor which isomer would you expect a greater equilibrium percentage of molecules with the alkyl group in the axial position, isopropylcyclohexane or propylcyclohexane? Explain. Isopropylcyclohexane Propylcyclohexanearrow_forwardDraw the four possible positions, the cis and trans positions of the methyl groups for both the chair and boat conformations, of 1,4-dimethylcyclohexane. Label each as cis or trans and identify the highest in energy.arrow_forward
- Draw all Newman projections of 2,2 bromopropane conformations in which the CH3 group and the H of the CHY2 group are positioned 'gauche' to each other.arrow_forwardHow do you account for the difference in energies between the two staggered conformations of 1,2-dichloroethane? How about for the two eclipsed conformations? Draw all four conformations and, on your drawing, indicate sources of strain – torsional, steric (gauche), steric eclipsed.arrow_forwardGg.194.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY