
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
10.) Would the base sodium phenoxide be a good choice for deprotonation of ethyl acetoacetate (pKa = 11)? Explain your answer fully.
b) Why is ethyl acetoacetate so acidic compared to the average ketone or ester, which have pKa values in the lower 20s?
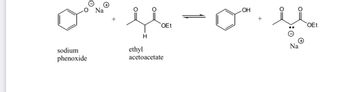
Transcribed Image Text:This image shows a chemical equilibrium reaction between sodium phenoxide and ethyl acetoacetate.
**Reactants:**
1. **Sodium Phenoxide (C₆H₅ONa)**
- Structure: A benzene ring with an oxygen anion (\(O^-\)) bonded to it, paired with a sodium cation (\(Na^+\)).
2. **Ethyl Acetoacetate (C₆H₁₀O₃)**
- Structure: Contains a ketone group, an ester group (\(OEt\)), and a methylene (\(CH_2\)) group.
**Products:**
1. **Phenol (C₆H₅OH)**
- Structure: A benzene ring with a hydroxyl group (\(OH\)) attached.
2. **Sodium Enolate of Ethyl Acetoacetate**
- Structure: The enolate form of ethyl acetoacetate, featuring a negatively charged oxygen (\(O^-\)) with sodium cation (\(Na^+\)).
The reaction is represented as an equilibrium process, indicating it can proceed in both forward and reverse directions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In an attempt to synthesize compound C through a two-step process, a chemist discovered after completing the first step that they had inadvertently produced two distinct compounds, A and B. Upon examining the infrared spectroscopy (IR) results, it was observed that both A and B exhibited peaks indicative of a ketone and an ester group. Please provide the molecular structures of A and B. OEt NaOEt ΕΙΟ A B In a chemical experiment, they noticed that both components, A and B, from a combined sample turned into a new compound, C, during the following stage. The task is to determine what compound C looks like and explain how compound A or B changes into compound C through a reaction. Compound C should be the primary molecule containing carbon created in this process, not just a by-product. A B H3O+, H₂O, A Mechanism = сarrow_forwardGive the name of the product formed when hydrochloric acid is mixed with N,N-dimethyl-2-propanamine.arrow_forwardCan any functional groups on Risperidone ionize? If so, which ones? Under what conditions? What are the pKa values of these functional groups? Write the structures in their correct ionization state at physiological pH.arrow_forward
- Which combination of steps would form the drug in the highest yield from the alcohol precursorarrow_forwardWrite the reactions for the preparation and properties of the following compoundsarrow_forwardThe hydrolysis of the ester shown here is catalyzed by morpholine. Explain how morpholine catalyzes the reaction. (Hint: The pKa of the conjugateacid of morpholine is 9.3, so morpholine is too weak a base to function as a base catalyst.)arrow_forward
- Assuming the reactions react, predict and label the conjugate acid and base pairs.arrow_forwardExplain the differences in pKa (vs. benzoic acid) of these substituted benzoic acids based on fundamental chemical principles (not just what pKa means).arrow_forwardThe catalog prices for all reagents used to make Phenacetin are shown: p-ethoyxanaline: 100 grams, $26.00 (Weight used: 2.5 grams) Acetic Anhydride: 1000 grams, $39.50 (Weight used: 2.2 grams, Density: 1.08) Hydrochloric Acid: 2500 mL, $30.20 (Weight used: 1.53 grams, Density: 1.02) Sodium Acetate Trihydrate: 500 grams, $30.50 (Weight used: 3.0 grams) Calculate the cost of each reagent.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY