
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
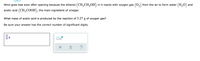
Transcribed Image Text:Wine goes bad soon after opening because the ethanol (CH,CH,OH) in it reacts with oxygen gas (0,) from the air to form water (H,O) and
acetic acid (CH3COOH), the main ingredient of vinegar.
What mass of acetic acid is produced by the reaction of 3.27 g of oxygen gas?
Be sure your answer has the correct number of significant digits.
g
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4.106. Aspirin (acetlsalicyclic acid) may be formed from salicyclic acid and acetic acid as follows:C7H6O3 (aq) + CH3COOH (aq) → C9H8O4(s) + H2O(l)Salicyclic acid Acetic Acid Aspirin b. How many mol of aspirin may be produced from 1.00*102 mol mol salicylic acid? c. How many g of aspirin may be produced from 1.00*102 mol salicylic acid? d. How many g of acetic acid would be required to completely with the 1.00*102 mol salicylic acid? e. For the conditions in part (d), how many g of aspirin would form?arrow_forwardSuppose a 250. mL flask is filled with 1.3 mol of CO, 1.0 mol of CO, and 1.8 mol of H,. This reaction becomes possible: 2 CO (g) +H,0(g) – CO,(g)+H,(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CO. You can leave out the M symbol for molarity. CO H20 co2 H2 initial ? change equilibriumarrow_forwardSuppose we react 4.00 mol of HCl with 1.00 mol of Ca,(PO,), to produce phosphoric acid, H,PO, 4 according to the balanced reaction, 6 HCl(aq) + Ca,(PO,),(s) → 2 H,PO,(aq) + 3 CaCl,(aq) What reactant remains when the other is fully consumed, and how many moles of this reactant remain? A 0.33 mol of Ca,(PO,)2 B 0.66 mol of Ca,(PO,), C 1.00 mol of HCl i D 3.00 mol of HClarrow_forward
- Suppose a 500. mL flask is filled with 0.60 mol of H,S, 0.10 mol of CS, and 1.5 mol of H,. This reaction becomes possible: CH (g) + 2H,S (g) =CS,(g)+4H, (g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CH4. You can leave out the M symbol for molarity. CHA H,S cs, H2 initial change equilibriumarrow_forwardSuppose a 250. mL flask is filled with 1.3 mol of H,S, 1.2 mol of CS, and 0.70 mol of H,. This reaction becomes possible: CH,(s) + 2H,S (g) – cs,(e)+ 4H,(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CH. You can leave out the M symbol for molarity. CH H,S Cs, H2 initial change equilibrium Olo X Oarrow_forwardIf 45.0 g of O2 are mixed with 6.70 g of H2 and the mixture is ignited, what massof water is produced?arrow_forward
- Please see photoarrow_forwardSuppose a 250. mL flask is filled with 0.10 mol of CH, 0.50 mol of H,O and 1.8 mol of H,. This reaction becomes possible: CH, (g) +H,0(g) = CO(g)+3H,(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CO. You can leave out the M symbol for molarity. dla CH, H,O CO H, initial change equilibriumarrow_forward4 Fe +3 O2 ---> 2 Fe2O3 How many litters of iron metal are needed to react completely with 4.35 mol oxygen gas?arrow_forward
- 3.4 A 5.17 g sample of vinegar is titrated with 0.018 M NaOH. If the vinegar requires 42.3 mL of the NaOH solution for complete reaction, what is the mass percentage of acetic acid in the vinegar? (i.e. calculate the % of CH3COOH in the vinegar) The reaction is: NaOH(aq) + CH3COOH(aq) H2O(l) + NaCH3COO (aq) M,(CH3COOH) = 60.0arrow_forwardSuppose a 500. mL flask is filled with 0.10 mol of H,O, 0.40 mol of CO, and 1.3 mol of H,. This reaction becomes possible: CO (g) + H,O(g) -Co,g)+H2(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of H,O. You can leave out the M symbol for molarity. CO H,0 со, H, initial ? change equilibriumarrow_forwardA sample of 0.75 g of hydrogen gas was obtained by reacting 32.5 g hydrochloric acid with 18.0 g magnesium. Mg(s) + 2 HCI (aq) --> MgCl₂ (aq) + H₂(g) What is the percent yield for this reaction? 100% 83% 41% 50%arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY