
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
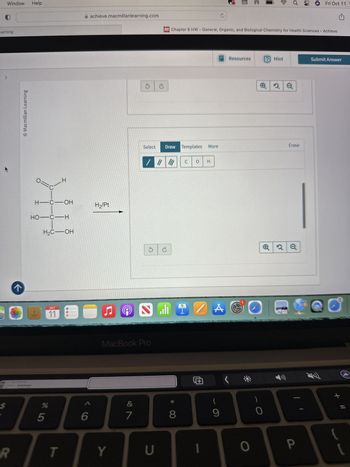
Transcribed Image Text:Window
Help
earning
k
>
Macmillan Learning
=
C
H
H-C-OH
HO-C-H
H2C-OH
x
(co
Fri Oct 11
A achieve.macmillanlearning.com
Chapter 6 HW - General, Organic, and Biological Chemistry for Health Sciences - Achieve
H2/Pt
Resources
? Hint
Submit Answer
2
Select
Draw Templates More
Erase
280
OCT
11
J
MacBook Pro
$
%
85
6
R
T
Y
5 G
C O H
&
*
(
7
8
9
U
A
>
0
0
2Q
P
+
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- answer only 3 and 4 thank you ill give tumbs up for the answerarrow_forwardChoose the coefficients that balance the following reaction: C3H5(NO3)3 → CO2 + N2 + H20 + 2:6:3:3:1 O 4:12:6:10:1 O 1:1:1:1:1:1 2:1:2:1:2 %23 %24 & backspace 21 3. 9. e r y tab a f k C V cularrow_forwardgenow.comiim/takeASSI [References) How many grams of sodium dichromate, Naz Cr207, should be added to a 250.0-mL volumetric flask to prepare 8.9 x 102 M Naz Crz 07 when the flask is filled to the mark with water? 6 item attempts remaining Submit Answer Try Another Version pt pt pt pt Previous Show Hint Email Instructor Cengage Learning | Cengage Technical Supportarrow_forward
- OF₂ (g) + H₂O(g) = O₂(g) + 2HF (9) [0₂] [2HF] [OF₂] [H₂O] O Kc = O K O Kc = Submit [OF 2] [H₂O] [0₂][HF] 2 [0₂][HF] 2 [OF ₂] [H₂O] Request Answerarrow_forwardX b Answered X Co. How to Ca X N NSU Logir x PeriodicTal X M Action Rec X 0 mySigTau X G 4.25 ml to X NBA Final X G scientific X+ now.com/ilrn/takeAssignment/takeCovalentActivity.do?locator%3Dassignment-take [References] Ethene is converted to ethane by the reaction C,H4 (9) + H2 (g) Catalyst + C2H6 (9) C2 H4 flows into a catalytic reactor at 25.6 atm and 280.°C with a flow rate of 1100. L/min. Hydrogen at 25.6 atm and 280.°C flows into the reactor at a flow rate of 1400. L/min. If 13.6 kg C2 Hg is collected per minute, what is the percent yield of the reaction? Percent yield Submit Answer Try Another Version 2 item attempts remaining Previous Next Email Instructor Save and Exit Cengage Learning | Cengage Technical Supportarrow_forwardnalysis Practice Worksheet.docx al Analysis Practice Worksheet.docx (13.5 KB) Page 1 > of 2 4. How much does a cubic meter of water weigh, in pounds? The density of water is 1.00 g/mL. SEP 10 étv MacBook Pro & 6. 7 %3D { R Y 60 * COarrow_forward
- Help 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward1. answer the following excersicearrow_forward20% D Safari File Edit View History Bookmarks Window Help Mon 12:47 PM 4+ G newconnect.mheducation.com My Questions | bartleby HW Set #39 (Ch1 3.8) Mail - Burgett, Alecia - Outlook ments HW Set #39 (Ch13.8) Saved Help Save & Exit Submit 3 3 attempts left Check my work cuments Click the "draw structure" button to launch the drawing utility. Report problem What monomer is used to form the following polymer? Hint еВok CH3 сH, СH, Solution nshots Print — сна— с — сн—с-сн—с 3 References Guided Solution ОСH-CH, Осн,сH3 ОсH-сH, draw structure... Mc Graw Hill 3 of 4 Prev Next NOV tv 25arrow_forward
- Ctrl Caps Shift M Inbox (1,543)-ftantill@udeledu x Mail-Francesca A Tantillo-Out x Homepage-CHM150-251 Chen X ← C - с app.101edu.co Question 19 of 20 Vitamin C (ascorbic acid, CeHsO6, 176.12 g/mol) can be measured by redox titration with iodine solution. lodine is reduced to iodide ion, while ascorbic acid is oxidized to dehydroascorbic acid (C6H6O6). The iodine solution is usually made in the presence of iodide ion, forming the more stable triiodide ion, Is, giving the following overall reaction CeHsOc(aq) + Is (aq) + H₂O(1)→ CHO(aq) + 31-(aq) + 2H+(aq) The titration is carried out in the presence of starch, which forms a dark blue complex with the excess iodine when the endpoint is reached. Ascorbic acid can be used to standardize the iodine solution. A 0.315 g sample of ascorbic acid was titrated with iodine solution, requiring 30.2 mL to reach the endpoint. Calculate [ls], the molar concentration of Is ion in the solution. O L Tab En Esc 79°F Sunny ! 1 Q A N FI @ 2 W S F2 X # 3 E D…arrow_forward||| O HYDROCARBONS Naming and drawing linear alkenes with one double bond Write the systematic name of each organic molecule: CI structure CI Explanation CI CI Check D 0 0 name MacBook Pro S © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use 0/5 | Privacy Carrow_forward100% 41 Safari File Edit View History Bookmarks Window Help Wed 4:51 PM A uk.instructure.com My Questions | bartleby Course Details University of Kentucky - CHE 111 Lab - Fall19 - FRENCH: Quiz.. Calculate the Molarity of Sodium Hydroxide? | Yahoo Answers = CHE111-017-032 > CHE111 Sections 017 to 32 (Tuesdays): General Chemistry I Laboratory (Fall 2019) Fall 2019 Home Account Announcements Dashboard Question #: 11 Syllabus Course Details An aqueous solution containing 0.3845 g of KHP (KHC;H4O4) was titrated with a solution of sodium hydroxide, producing the following titration curve. What is the concentration of the NaOH solution? Courses Modules Titration of KHP with NaOH Piazza Groups 14 Grades 12 Calendar 10 Inbox 6. Help Volume NaOH (ml) DEC 18arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY