
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
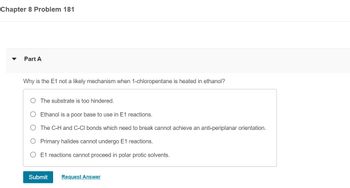
Transcribed Image Text:Chapter 8 Problem 181
Part A
Why is the E1 not a likely mechanism when 1-chloropentane is heated in ethanol?
The substrate is too hindered.
Ethanol is a poor base to use in E1 reactions.
The C-H and C-CI bonds which need to break cannot achieve an anti-periplanar orientation.
Primary halides cannot undergo E1 reactions.
E1 reactions cannot proceed in polar protic solvents.
Submit
Request Answer
![Part B
2-Bromobutane is treated with sodium methoxide in methanol at 323 K. Draw the major product of the reaction. Make sure to consider the stereochemistry of the reaction.
NN
IL
[1]
A
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.
► View Available Hint(s)
Submit
H: ²D EXP.¹ CONT
L
Marvin JS
by ChemAxon
4
H
C
N
O
S
CI
Br
I
Р
PE
Br
F
+
CH₂O-Na+
CH3OH
100 °C](https://content.bartleby.com/qna-images/question/0d37489c-b06e-4663-91d7-ac16cfad329d/7ed7376d-7d9c-4f93-97c5-700449e3300d/wnxous_thumbnail.jpeg)
Transcribed Image Text:Part B
2-Bromobutane is treated with sodium methoxide in methanol at 323 K. Draw the major product of the reaction. Make sure to consider the stereochemistry of the reaction.
NN
IL
[1]
A
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.
► View Available Hint(s)
Submit
H: ²D EXP.¹ CONT
L
Marvin JS
by ChemAxon
4
H
C
N
O
S
CI
Br
I
Р
PE
Br
F
+
CH₂O-Na+
CH3OH
100 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Wolff - Kishner reduction of 7-methyl-2-cycloheptenone produces 3-methylcycloheptene, not 4-methylcycloheptene. Write the reaction and its mechanism. It is not necessary to write a mechanism for the formation of the hydrazone. ANSER MUST BE DRAWN! Thank you.arrow_forwardTreatment of the following stereoisomer of 1-bromo-1,2- diphenylpropane with sodium ethoxide in ethanol gives a single stereoisomer of 1,2-diphenylpropene. H3C H C6H5 H C6H5 Br ***I CH3CH₂O Na+ Draw the E2 elimination product of the reaction. Take into account that the starting stereochemistry affects the resulting double bond stereochemistry. CH₂CH₂OH Consider E/Z stereochemistry of alkenes. Do not show stereochemistry in other cases. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. Sn [F ?arrow_forwardOne of the products that results when 1-bromo-2,2-dimethylcyclopentane is heated in ethanol is shown below. Propose a mechanism by which it is formed.arrow_forward
- Give a mechanism for the following reaction.arrow_forwardPlease don't provide handwritten solution ...arrow_forward6. Consider the following reaction scheme: 1) l2, NAOH, H,0 2 OH + ICH3 2 2) H30* Draw a detailed mechanism with arrows showing the movement of electrons for the formation of benzoic acid and iodoform from 1,3-diphenyl-1,3-propanedione (2).arrow_forward
- 2) Provide the reagent(s) and the reaction mechanism of the following reaction. 1 (* is the radio labeled carbon 14C)arrow_forward-45 In the presence of a small amount of bromine, the following light-promoted reaction has been observed. CH3 H₂C CH3 H₂C. hv + Br₂ CH3 + Br H₂C. H Br (a) Write a mechanism for this reaction. Your mechanism should explain how both products are formed. (Hint: Notice which H atom has been lost in both products.) (b) Explain why only this one type of hydrogen atom has been replaced, in preference to any of the other hydrogen atoms in the starting material. amamber that bromination is highly selectivearrow_forwardPls solve this question correctly instantly in 5 min i will give u 3 like for surearrow_forward
- 2-chloro-3-methylpentane is reacted with water in an E1 reaction. What is the IUPAC name of the single major expected E1 product of this reaction? Do NOT include stereochemistry (E/Z) in the name. The name must be correctly spelled and properly formatted (e.g. dashes, spaces, numbers, etc) according to IUPAC rules for credit.arrow_forwardWhich mechanism accomplishes this transformation? O O bla A B D Ar + (A) (0) H Ar (B) (D) H3C H A quesese H CH3 Ar + HO Ararrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY