
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
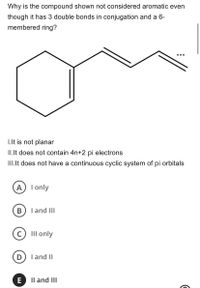
Transcribed Image Text:Why is the compound shown not considered aromatic even
though it has 3 double bonds in conjugation and a 6-
membered ring?
•..
I.It is not planar
II.Ilt does not contain 4n+2 pi electrons
III.It does not have a continuous cyclic system of pi orbitals
A I only
B
I and III
III only
I and II
E
Il and III
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Name these molecules and determine if they are aromatic according to Huckel's Rule (a) (b) Aromatic? Aromatic? (c) (d) Aromatic? plas Aromatic?arrow_forward5.) How many stereogenic centers are present in the following compound derived from citrus oils? А. 1 В. 3 С. 4 D. 5 Keywords: chiral, asymmetric carbon, stereogenic center, nonequivalent group, sp', tetrahedral, sp², sp, trigonal planar, linear Concepts: stereoisomerism, asymmetric carbons 6.arrow_forwardDraw an 8 membered ring that is aromatic and draw the molecular orbital diagram derived from frosts circlearrow_forward
- A. B. C. Me CH3 ClC H ¿ OH NH₂ H Br-C-C H3C H Br CH3 Comparing structure pair in B OH Comparing structure pair in C [Select] Me HC CI Comparing structure pair in A [Select] [Select] Same structure drawn differently Enantiomers Diastereomers NH₂ Br H3C CH3 Br HC-CCH3 H For the following two structures compared in parts A and B, determine whether they are enantiomers OR diastereomers OR the same structure drawn differently (non-bonding electrons not included for clarity).arrow_forwardAZT was the first drug approved to treat HIV, the virus that causes AIDS. Explain why the sixmembered ring of AZT is aromatic.arrow_forwardCompound 1 Br Bri НІ T ... H The correct IUPAC names are: Compound 2 H Br Br I.. H Br The compounds are diastereomers not isomeric enantiomers identical constitutional isomers Compound 1: (2R, 3R)-1,2,3-tribromobutane, Compound 2: (2S, 3R)-1,2,3-tribromobutane Compound 1: (2R, 3S)-1,2,3-tribromobutane, O Compound 1: (2R,3R)-1,2,3-tribromobutane, Compound 2: (2S,3S)-1,2,3-tribromobutane Compound 2: (2R, 3R)-1,2,3-tribromobutane O Compound 1: (2S,3S)-1,2,3-tribromobutane, Compound 2: (2R,3S)-1,2,3-tribromobutanearrow_forward
- 6. Consider each compound below. Note the number of T electrons in the ring in each compound and indicate if it is aromatic or not aromatic (assume planarity for all molecules). B. TT electrons TT electrons TT electrons Aromatic? Aromatic? Aromatic?arrow_forwardNonearrow_forwardAZT was the rst drug approved to treat HIV, the virus that causes AIDS. Explain why the sixmembered ring of AZT is aromatic.arrow_forward
- q12 PLS HELP VERY URGENT, NO EXPLANATION REQUIREDarrow_forward14. Menthol is a mild anasthetic isolated from peppermint oil. The most common stereoisomer is shown at left in bond line. Which chair structure represents menthol? SOH A) B) CH3 CH3 OH by OH CH(CH3)2 CH(CH3)2 C) D) OH CH3 IN OH ОН CH3 CH(CH3)2 CH(CH3)2arrow_forwardAre each of the following molecules aromatic, antiaromatic or non-aromatic? A. I: aromatic, II: antiaromatic, III: aromatic, IV: non-aromatic, V: non-aromaticB. I: aromatic, II: antiaromatic, III: aromatic, IV: aromatic, V: non-aromaticC. I: aromatic, II: aromatic, III: antiaromatic, IV: non-aromatic, V: non-aromaticD. I: antiaromatic, II: aromatic, III: aromatic, IV: non-aromatic, V: antiaromaticE. I: antiaromatic, II: aromatic, III: non-aromatic, IV: non-aromatic, V: aromaticarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY