
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Why are these chiral centers when it is connected to two Carbons so not 4 different groups?
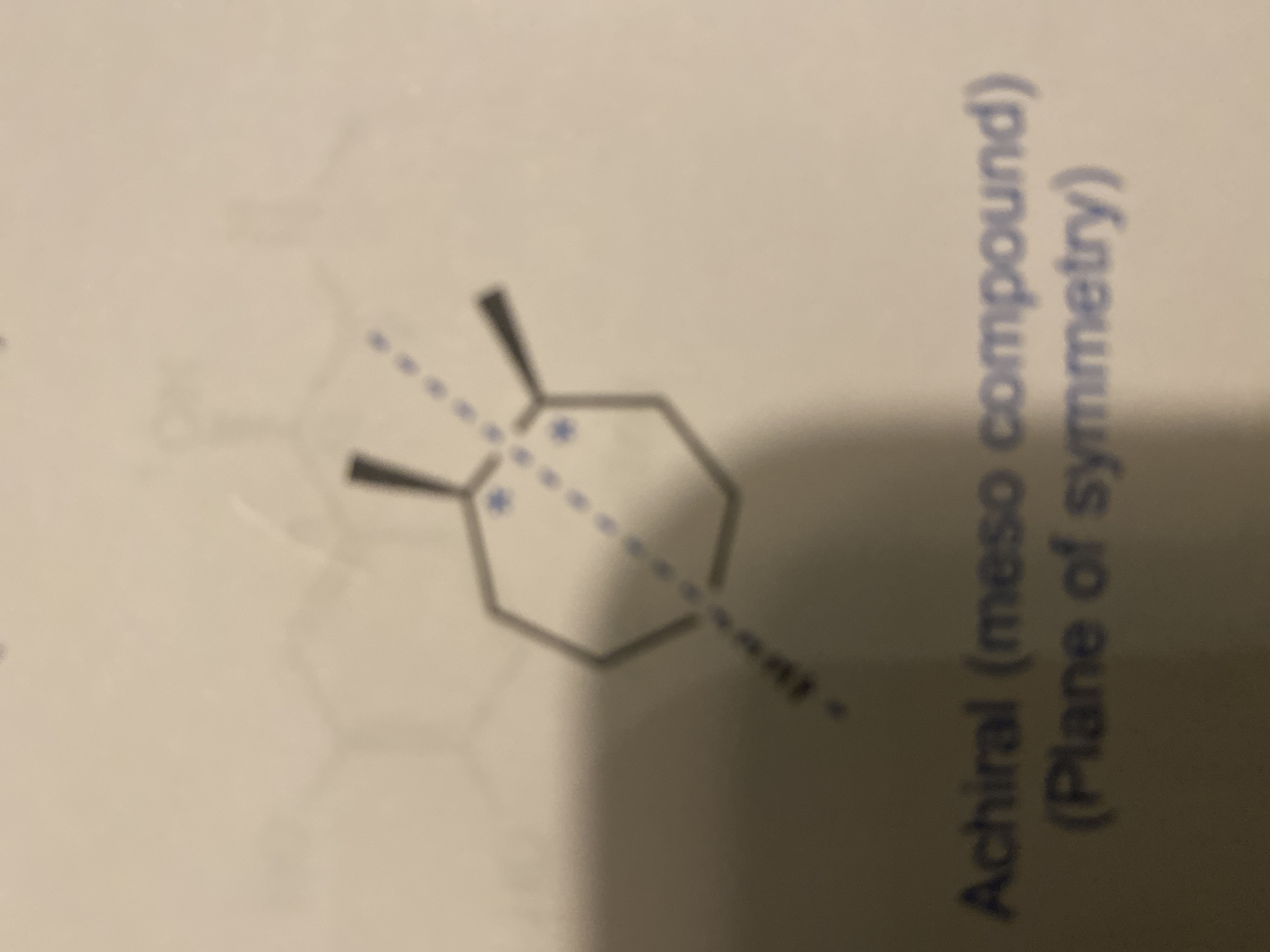
Transcribed Image Text:### Understanding Meso Compounds
**Diagram Analysis:**
The image presents a chemical structure illustrating an achiral (meso) compound. The compound is depicted with a six-membered ring featuring two substituents, each marked with a wedge line to indicate stereochemistry. A dashed blue line runs vertically through the center of the ring, representing the plane of symmetry.
**Key Points:**
1. **Meso Compounds:**
- Meso compounds are a type of stereoisomer that appear chiral due to the presence of stereocenters but are actually achiral due to an internal plane of symmetry.
- Despite having multiple stereocenters, the molecule is superimposable on its mirror image, making it achiral.
2. **Plane of Symmetry:**
- The dashed line indicates the plane of symmetry, which divides the molecule into two mirror-image halves.
- The presence of this plane is the critical factor that renders the compound achiral.
Understanding meso compounds is essential in stereochemistry as they provide insights into molecular symmetry and chiral behavior, despite complex appearances due to multiple stereocenters.
Expert Solution
arrow_forward
Step 1
Given : Structure of Cis 1,2 dimethyl cycloheptane
To find : Chiral center
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 5. Two stereoismers with two chiral stereogenic centers that are not mirror images are:arrow_forward.. O 2. Give the molecula formula for the following compounds and star the chiral center(s) in each one of them if there is any. HO NO2 HS N' HOOJ HN Но H. CH3 followingnotegu F. HOO) HO, CH3 HOO). N. ఆరింది 3. Define chiral and an achiral molecules. 4. Define structural (or constitutional isomers). Draw as many constitutional isomers as possible with a molecular formula of C6H14 5. Define stereoisomers and give an example of two stereoisomers (draw them in line-angle notation). 6. Define diastereomers and give an example of two diastereomers (draw them in line-angle notation). 7. Define enantiomers and give an example of two enantiomers (draw them in line-angle notation).arrow_forwardPlease don't provide handwriting solutionarrow_forward
- Compounds that have non-superimposable mirror images are (select all possible answers) chiral. optically active. enantiomers. meso. identical. achiral.arrow_forwardIdentify which of the compound is chiral.a. T-butyl alcoholb. 2,2-dimethylpropanec. 1,2,4,5-tetramethylcyclohexaned. 2-hydroxypropanoic acidarrow_forwardWhat are the configurations of all chiral centers of the attached molecule? Please state the answer in clear words along with any pictures/reactions.arrow_forward
- 2. For the following pairs of molecules, determine if their relationship is enantiomers, diastereomers, or the same compound. Assign R/S configuration to all chiral centers, if applicable. ➤ > CH₂ H₂CH.. off offarrow_forwardSolve the given question #3arrow_forward2. If a molecule has 3 asymmetric carbon centers, how many stereoisomers are there? Show work. ^.arrow_forward
- Please don't provide handwritten solution...arrow_forwardCan you explain to me about the chiral carbon because I don't get it at all? Also can you give me an example of the chiral carbon, please?arrow_forward15. Build two different models of 2-butene. This is a four-carbon chain with a double bond between carbons 2 and 3. a. These two models are stereoisomers. What is their relationship? Enantiomers, diastereomers, something else? b. Classify the two models as cis- and trans-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY