
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Scientific question:
Why are compounds composed of integer ratios of elements? If the charges are not balanced it cannot be correct which is what we see in the image attached. Charges must be neutral/zero.
The image in itself will help answer the question above.
Note: You're answering the question above not the question in the image attached.
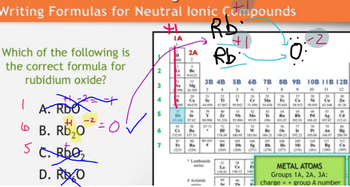
Transcribed Image Text:Writing Formulas for Neutral lonic Compounds
Which of the following is
the correct formula for
rubidium oxide?
1
ما
A. RDO
H
B. RD₂0-0
5 C. R50₂
D. RO
2
3
4
5
6
7
اید
ΤΑ
1-² 0-1
2A
9490122
Mg
13291 137.33
Rb.
Rb
Lanthanide
#Actinide
3B 4B 5B 6B 7B 8B 9B 10B IIB 12B
4
5
10
12
2628SRCS&L
4007 44.95647367 50.942 51.996 54.938 55.845 58.933 58.5 63.546
4538
41
48
762906 91224 92.906 45.95 (96) 1007 102.91 1064210737 11241
O
2
(MD) (270)
27
178.49 1805 183.84 186.21 190.23 192.22 1950 196.97 300.39
110 III 112
(285)
METAL ATOMS
Groups 1A, 2A, 3A:
charge = + group A number
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution ....show work..don't give Handwritten answer..don't use Ai for answering thisarrow_forwardIn an alien world, a different periodic table exists. It is organized similar to the periodic table in our world, just smaller. The first group contains the alkali metals, the second group the alkaline earth metals, there are a few transition metals, and then some non-metals. The periodic table is shown below in picture form, and a table is given with molar masses and common ionic charges. Also in the table is a list of common ion groups (similar to carbonate, nitrate, etc. in our world.) Below is a table showing the molar masses and common ionic charges Element Symbol Atomic Number Atomic Mass Common Charge(s) FixedOrVariable Element or Group Name of anion Hydrogen H 0 Jellium J 1 1.28 2 Fixed Element Dogium D 2 2.48 -3 Fixed Element Dogide Gorillium G 3 4.87 -2 Fixed Element Gorillide Monkium M 4 7.27 -1 Fixed Element Monkide Jucium Oj 5 9.39 1 Fixed Element Racium R 6 11.18 2 Fixed Element Romium Ro 7 14.05 -3 Fixed Element Romide Julietium Ju…arrow_forwardPlease help with this.arrow_forward
- 13 In an alien world, a different periodic table exists. It is organized similar to the periodic table in our world, just smaller. The first group contains the alkali metals, the second group the alkaline earth metals, there are a few transition metals, and then some non-metals. The periodic table is shown below in picture form, and a table is given with molar masses and common ionic charges. Also in the table is a list of common ion groups (similar to carbonate, nitrate, etc. in our world.) Below is a table showing the molar masses and common ionic charges Element Symbol Atomic Number Atomic Mass Common Charge(s) FixedOrVariable Element or Group Name of anion Hydrogen H 0 Jellium J 1 1.28 2 Fixed Element Dogium D 2 2.48 -3 Fixed Element Dogide Gorillium G 3 4.87 -2 Fixed Element Gorillide Monkium M 4 7.27 -1 Fixed Element Monkide Jucium Oj 5 9.39 1 Fixed Element Racium R 6 11.18 2 Fixed Element Romium Ro 7 14.05 -3 Fixed Element Romide Julietium…arrow_forwardWrite the correct formulas and balance the equations:A. Gallium plus perchloric acid yields gallium perchlorate plus water plus dichlorine pentoxide. (Note: the coefficient of the water is 9 and the coefficient of dichlorine pentoxide is 3.) B. Aluminum hydroxide plus sulfuric acid yields aluminum sulfate plus water.arrow_forwardA 3.000 g sample of a gaseous compound was found to contain 2.560 g of carbon and 0.440 g of hydrogen. A) What is the simplest formula of the compound (Write C first and then H)? You won't be able to make the numbers a subscript. For instance, for MgCl2 enter MgCl2. B) The molar mass of the compound was found to be 42.08 g/mol. What is the molecular formula of the compound? You won't be able to make the numbers a subscript. For instance, for MgCl2 enter MgCl2.arrow_forward
- 14 In an alien world, a different periodic table exists. It is organized similar to the periodic table in our world, just smaller. The first group contains the alkali metals, the second group the alkaline earth metals, there are a few transition metals, and then some non-metals. The periodic table is shown below in picture form, and a table is given with molar masses and common ionic charges. Also in the table is a list of common ion groups (similar to carbonate, nitrate, etc. in our world.) Below is a table showing the molar masses and common ionic charges Element Symbol Atomic Number Atomic Mass Common Charge(s) FixedOrVariable Element or Group Name of anion Hydrogen H 0 Jellium J 1 1.28 2 Fixed Element Dogium D 2 2.48 -3 Fixed Element Dogide Gorillium G 3 4.87 -2 Fixed Element Gorillide Monkium M 4 7.27 -1 Fixed Element Monkide Jucium Oj 5 9.39 1 Fixed Element Racium R 6 11.18 2 Fixed Element Romium Ro 7 14.05 -3 Fixed Element Romide Julietium…arrow_forwardWhat is an empirical formula? What is a molecular formula? Do ionic compounds have molecular formulas?arrow_forward27 In an alien world, a different periodic table exists. It is organized similar to the periodic table in our world, just smaller. The first group contains the alkali metals, the second group the alkaline earth metals, there are a few transition metals, and then some non-metals. The periodic table is shown below in picture form, and a table is given with molar masses and common ionic charges. Also in the table is a list of common ion groups (similar to carbonate, nitrate, etc. in our world.) Below is a table showing the molar masses and common ionic charges Element Symbol Atomic Number Atomic Mass Common Charge(s) FixedOrVariable Element or Group Name of anion Hydrogen H 0 Jellium J 1 1.28 2 Fixed Element Dogium D 2 2.48 -3 Fixed Element Dogide Gorillium G 3 4.87 -2 Fixed Element Gorillide Monkium M 4 7.27 -1 Fixed Element Monkide Jucium Oj 5 9.39 1 Fixed Element Racium R 6 11.18 2 Fixed Element Romium Ro 7 14.05 -3 Fixed Element Romide Julietium…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY