
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Chemistry
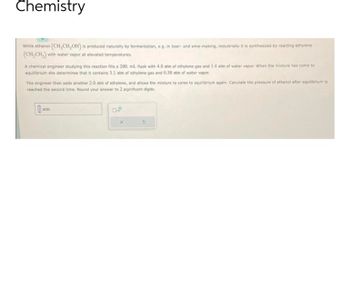
While ethanol (CH₂CH₂OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene
(CH₂CH₂) with water vapor at elevated temperatures.
A chemical engineer studying this reaction fills a 200. mL flask with 4.0 atm of ethylene gas and 1.4 atm of water vapor. When the mixture has come to
equilibrium she determines that it contains 3.1 atm of ethylene gas and 0.50 atm of water vapor.
The engineer then adds another 2.0 atm of ethylene, and allows the mixture to come to equilibrium again. Calculate the pressure of ethanol after equilibrium is
reached the second time. Round your answer to 2 significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- While ethanol (CH,CH,OH)is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH,CH,) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 500. mL flask with 2.3 atm of ethylene gas and 4.3 atm of water vapor. When the mixture has come to equilibrium he determines that it contains 1.57 atm of ethylene gas and 3.57 atm of water vapor. The engineer then adds another 2.2 atm of water, and allows the mixture to come to equilibrium again. Calculate the pressure of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. atmarrow_forwardWhile ethanol (CH₂CH₂OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH₂CH₂) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 2.0 L flask with 4.7 atm of ethylene gas and 1.6 atm of water vapor. When the mixture has come to equilibrium she determines that it contains 3.5 atm of ethylene gas and 0.40 atm of water vapor. The engineer then adds another 2.4 atm of ethylene, and allows the mixture to come to equilibrium again. Calculate the pressure of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. atm 33 0 x10 X S 114 ype URL 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility CH151 152_lab....docx ☆ ^ W + Chapter 13- Che....doc Showarrow_forwardWhile ethanol (CH,CH,OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH,CH,) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 200. mL flask with 1.7 atm of ethylene gas and 2.2 atm of water vapor. When the mixture has come to equilibrium he determines that it contains 0.92 atm of ethylene gas and 1.42 atm of water vapor. olo Ar The engineer then adds another 1.1 atm Of water, and allows the mixture to come to equilibrium again. Calculate the pressure of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. atmarrow_forward
- As you are walking across your laboratory, you notice a 5.25 L flask containing a gaseous mixture of 0.0205 mole NO2 (9) and 0.750 mol N2O4 (q) at 25°C. 4 (g) Is this mixture at equilibrium? If not, will the reaction proceed towards forming more products, or more reactants? N2O4 4 (9) → 2NO2 (9) Ko = 4.61 x 103 at 25°Carrow_forwardWhile ethanol (CH3CH2OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH2CH2) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 1.5 L flask with 4.8 atm of ethylene gas and 3.9 atm of water vapor. When the mixture has come to equilibrium she determines that it contains 3.1 atm of ethylene gas and 2.2 atm of water vapor. The engineer then adds another 1.2 atm of ethylene, and allows the mixture to come to equilibrium again. Calculate the pressure of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. at atm 5arrow_forwardthis one is asking for atmarrow_forward
- While ethanol (CH,CH,OH) is produced naturally by fermentation, e.g. in beer- and wine-making, industrially it is synthesized by reacting ethylene (CH,CH,) with water vapor at elevated temperatures. A chemical engineer studying this reaction fills a 500. mL flask with 2.9 atm of ethylene gas and 1.7 atm of water vapor. When the mixture has come to equilibrium she determines that it contains 2.14 atm of ethylene gas and 0.94 atm of water vapor. The engineer then adds another 0.97 atm of ethylene, and allows the mixture to come to equilibrium again. Calculate the pressure of ethanol after equilibrium is reached the second time. Round your answer to 2 significant digits. atmarrow_forwardWhen exposed to UV light, phosgene (COCL2) decomposes according to the reaction COCL2(g)->CO(g) + Cl2(g). All the air is removed from a flask. The flask is then filled with phosgene to a pressure of 2.8 atm and then exposed to UV light. If the temperature remains constant during the decomposition, what is the partial pressure of Cl2 (g) in the flask after the decomposition is complete, assuming 100% yield?arrow_forwardNitrogen dioxide is one of the many oxides of nitrogen (often collectively called "NOX") that are of interest to atmospheric chemistry. It can react with itself to form another form of NOx, dinitrogen tetroxide. A chemical engineer studying this reaction fills a 2.0 L flask with 2.9 atm of nitrogen dioxide gas. When the mixture has come to equilibrium he determines that it contains 1.4 atm of nitrogen dioxide gas. The engineer then adds another 1.5 atm of nitrogen dioxide, and allows the mixture to come to equilibrium again. Calculate the pressure of dinitrogen tetroxide after equilibrium is reached the second time. Round your answer to 2 significant digits. atm ☐ x10 Xarrow_forward
- Nitrogen dioxide is one of the many oxides of nitrogen (often collectively called "NOx") that are of interest to atmospheric chemistry. It can react with itself to form another form of NOx, dinitrogen tetroxide. A chemical engineer studying this reaction fills a 125.L tank at 13.°C with 16.mol of nitrogen dioxide gas. He then raises the temperature considerably, and when the mixture has come to equilibrium determines that it contains 6.9mol of nitrogen dioxide gas. The engineer then adds another 8.0mol of nitrogen dioxide, and allows the mixture to come to equilibrium again. Calculate the moles of dinitrogen tetroxide after equilibrium is reached the second time. Round your answer to 2 significant digits.arrow_forwardNitrogen dioxide is one of the many oxides of nitrogen (often collectively called "NOx") that are of interest to atmospheric chemistry. It can react with itself to form another form of NOx, dinitrogen tetroxide. A chemical engineer studying this reaction fills a 2.0 L flask with 1.0 atm of nitrogen dioxide gas. When the mixture has come to equilibrium she determines that it contains 0.34 atm of nitrogen dioxide gas. The engineer then adds another 0.25 atm of nitrogen dioxide, and allows the mixture to come to equilibrium again. Calculate the pressure of dinitrogen tetroxide after equilibrium is reached the second time. Round your answer to 2 significant digits. atm x10 5arrow_forwardPlease answer it correctlyarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY