
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
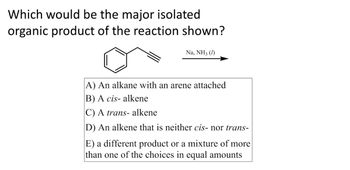
Transcribed Image Text:**Question:**
Which would be the major isolated organic product of the reaction shown?
**Reaction Setup:**
- Reactants: A benzene ring attached to a terminal alkyne
- Reagents: Na, NH₃ (liquid)
**Options:**
A) An alkane with an arene attached
B) A cis- alkene
C) A trans- alkene
D) An alkene that is neither cis- nor trans-
E) A different product or a mixture of more than one of the choices in equal amounts
**Explanation:**
This image likely depicts a partial reduction reaction of an alkyne using sodium in liquid ammonia, also known as the Birch reduction. This method typically converts alkynes to trans-alkenes. Hence, option C may represent the major product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chemistryarrow_forwardDraw the structural formula for the product formed upon hydroboration/oxidation of the Alkene below.arrow_forwardDraw a structural formula for the major organic product of the following reaction: -CH=CH₂ + Br₂ ***** • Show product stereochemistry IF the reactant alkene has both carbons of the double bond within a ring. • Do not show stereochemistry in other cases. • If enantiomers are formed, just draw one. Sn [1 CH₂Cl₂ ChemDoodle Previous Nextarrow_forward
- Draw a structural formula for the substitution product of the reaction shown below. H3C CI CH3OH • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If more than one stereoisomer of product is formed, draw both. • Separate multiple products using the + sign from the drop-down menu.arrow_forwardQuestion 6 Give the major organic product for the following reaction. HgS0, CH3(CH,);C=CH O Cyclohexane 2-Hexanone O Cyclopentanol O Hexanal O Hexanolarrow_forwardThe reaction of propan-2-ol in the following series of reactions will yield __________. 1) NaH; then set aside until Step 3; 2) CH4 plus Br2, light and heat, assume monobromination; 3) the product of Step 1 mixed with the product of Step 2 an alkene an alkoxide an acetylide an ether an alkyl bromidearrow_forward
- Chemistry the question numbers are in the top left corner of each picture. some questions have more than one picture. thanks!!!arrow_forward3. Draw structural formulas for the major product(s) resulting from the following reactions: OH Cl2 (a) FeCl3 CH3 .CH3 KMNO4, H* Heat (b) Он (c) Br2arrow_forwardQuestion 2 Which statement is consistent with the Hammond postulate? O the transition state of an endothermic reaction step structurally resembles the reactant. O it is used to assign the E/Z stereochemistry of alkenes. the less stable carbocation intermediate is formed in electrophilic additions to alkenes. O in additions of HX to alkenes, the H adds to the less substituted alkene carbon and the X adds to the more substituted alkene carbon. O the transition state of an exothermic reaction step structurally resembles the reactant. A Moving to another question will save this response. 80 F3 54 $ 4 000 000 F4 % 5 F5 6 MacBook Air F6 & 7 ao F7 8 DII F8 ( 9 F9arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY