
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working
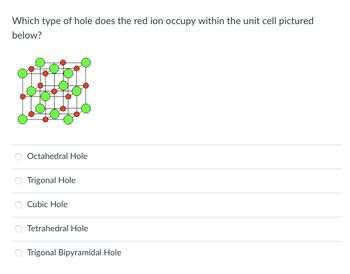
Transcribed Image Text:Which type of hole does the red ion occupy within the unit cell pictured
below?
Octahedral Hole
Trigonal Hole
Cubic Hole
Tetrahedral Hole
Trigonal Bipyramidal Hole
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- em 119 My Course X Macmillan: X Course Mo Submit Answer X Sections 5 What is the FORMULA for the limiting reagent? References X age.com/static/nb/ui/evo/index.html?deploymentid=5735112480241329813180832311&elSBN=9781305862883&id=1707786042&snapsh What amount of the excess reagent remains after the reaction is complete? HOMEWOR Use the References to access Important values if needed for this question. For the following reaction, 20.3 grams of carbon dioxide are allowed to react with 39.6 grams of potassium hydroxide. carbon dioxide (g) + potassium hydroxide (aq) potassium carbonate (aq) + water (1) - What is the maximum amount of potassium carbonate that can be formed? MacBook Air X grams MindTap - grams ;arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- Content Google Do google slid X Dr. Ortiz a ☑ Certificate ☑ > Course EX Cengage L ☑ OWLv2 | O ✓ Search res ChatGPT ✓ + C prod03-cnow-owl.cengagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator-assignment-take New Chrome available : E4 CH 16 17 and 18 [References] Question 1 1 pt Question 2 2 pts "Heater Meals" are food packages that contain their own heat source. Just pour water into the heater unit, wait a few minutes, and voilà! You have a hot meal. Mg(s) + 2 H2O(l) → Mg(OH) 2 (s) + H2(g) Question 3 1 pt Species AH° (kJ/mol) S° (J/mol·K) AƒG° (kJ/mol) Question 4 2 pts Mg(s) 0 32.67 0 Question 5 1 pt Question 6 × 2 pts H2O(l) Mg(OH)2(s) -285.83 69.95 -237.15 -924.54 63.18 -833.51 H2(g) 0 130.7 0 Question 7 1 pt Question 8 2 pts Question 9 1 pt HEATER MEALS Question 10 2 pts Question 11 1 pt Question 12 1 pt Question 13 Charles D. Winters INCLUDES 34 ou b HEATER MEALS 1 pt Question 14 1 pt The heat for the heater unit is produced by the reaction of magne- sium with water.…arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- 2+ d. 4H3O+ (aq) + 2Cl(aq) + MnO₂ (s) ⇒ Mn²+ (aq) + 6H₂O(1) + Cl₂ (9) Oke O Ke = = O Ke = 2+ [Mn²+ ][C1₂] [H3O+] * [CI-1² [Mn²+][H₂O][C1₂] 2+ [H³O+][CI¯]²[MnO₂2] [Mn²+ ] [H₂O1] [C1₂] 2+ [H3O+] * [CI-1²[MnO₂]arrow_forwardsession.masteringchemistry.com/myct/itemView?assignment ProblemID=190556628&offset=next Discussion Question 1: Chapter 6 Homework blem 6.20- Enhanced - with Feedback Enter the symbols for the ions and the correct formula for the onic compound formed by each of the following. esc Q A N F1 2 W S F2 X #3 20 E D F3 с S4 $ OOO 000 R Part E F4 F Enter the symbols for the ions of sodium and phosphorus. Enter the lons formed by these elements and separate your answers with a comma (e.g., Sr²+, As³-). cation, anion = Part F Complete previous part(s) Submit Part G cation, anion Submit Part H Complete previous part(s) 65° V Enter the symbols for the ions of calcium and sulfur. Enter the lons formed by these elements and separate your answers with a comma % Request Answer T Request Answer G 6 ΑΣΦ B ΑΣΦ MacBook Air F6 Y H 18⁰ h & 7 N F7 U * CO 8 J BUT RAS poddany ▶II ? F8 1 ? E 9 K F9 O L (e.g., Sr²+, As³-). 0 Ga3+ and 02-E L F10 P F11arrow_forward3:15 PM P w 99+ 4/12/2021 AutoSave Carbox. Acids, Derivatives & Amines-Sp 21.doc - Compatibility Mode - Word cyrine joshan lopez ff CJ File Home Insert Draw Design Layout References Mailings Review View Help A Share P Comments O Find S Replace - A A Aav A EE • E E E ¶ AaBbCcD AaBbCcDc AABBC AaBbCc Times New Romai 12 Paste BIU v ab x, x A e A 三三。 1 Normal 1 No Spac... Heading 1 Heading 2 A Select v Dictate Sensitivity Editor Reuse Files Clipboard N Font Paragraph Styles Editing Voice Sensitivity Editor Reuse Files Navigation Search document 8. What is the name of the following compound? Headings Pages CH2CH3 Results <-CH,CH, ci° ČH2CH3 Create an interactive outline of your document. c. N,N,N-triethylanilinium chloride d. N,N,N-triethylaniline chlorine It's a great way to keep track of where you are or quickly a. N,N,N-trimethylanilinium chloride b. N,N,N-diethylanilinium chloride move your content around. To get started, go to the Home tab and apply Heading styles to the headings in…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY