
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Which subshell fills immediately after the 5d subshell?
Expert Solution
arrow_forward
Step 1: Aufbau principle:
According to this principle orbitals are filled in the increasing order of their energy.
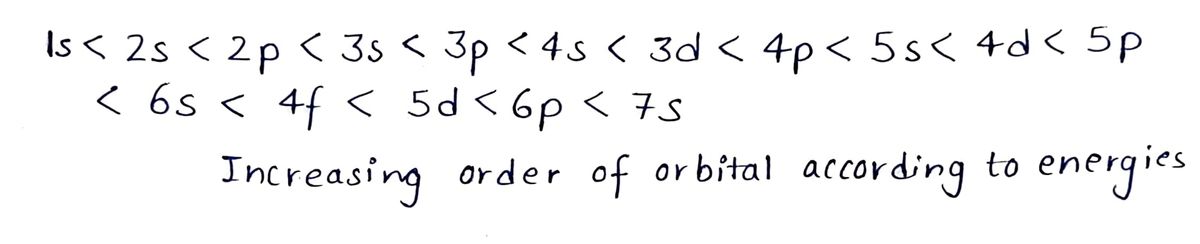
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the subshell designations are possible and which are impossible? Possible Impossible 5f 4d Op 2f 1d Answer Bankarrow_forwardHow many unpaired electrons are present in an atom with 32 electrons?arrow_forwardWhich type of electromagnetic radiation has the longest wavelength? red light blue light gamma rays x rays microwavesarrow_forward
- Which form of electromagnetic radiation has photons with the lowest energy Radio waves microwaves x-rays gamma rays or infrared radiationarrow_forwardWhat is the maximum number of electrons that a single d subshell can hold?arrow_forward19.) Which subshell can "accommodate" 6 electrons?arrow_forward
- Which form of electromagnetic radiation has photons with the highest energy? Radio Waves Infrared Radiation Gamma Rays O X-rays Microwavesarrow_forwardWhat is the total number of electrons in spherically shaped orbitals in an atom of Cs?arrow_forwardAccording to Aufbau principle, the 2p orbital should appear after which orbital? 1s B 2s C) 3s 4sarrow_forward
- using the aufbau principle , indicate how many electrons a 7s subshell can hold select the correct answer : 14 10 2 6arrow_forward12) Consider element 16, S. Draw a chart that depicts the relative energies of the first 6 ionization energies for S. Describe why you drew these ionization energies at the relative levels.arrow_forwardWhat other subshells are allowed in the lowest energy level that contains a d subshell? 3s 3p 3f 4s 4p What other subshells are allowed in the lowest energy level that contains an f subshell? 4s 4p 4d 3d 5darrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY