
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
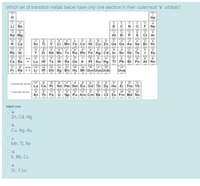
Transcribed Image Text:Which set of transition metals below have only one electron in their outermost "s" orbitals?
2
H
Не
400
ogen
en
6
10
Li Be
CN
OFNE
201
magstn
12
phonptone
15
thme
arge
11
13
14
16
17
18
Na Mg
AI
Si
P
S
ci Ar
gem
32
onme
emm
n
19
20
21
22
23
24
25
26
27
28
29
30
31
33
34
35
36
K Ca
Sc Ti
v Cr Mn
Fe Co Ni
Cu Zn Ga Ge As Se Br Kr
201
in
nitetn
37
romn
tey
ode
38
39
40
41
42
43
44
45
46
47
48
49
54
Rb Sr
Y Zr Nb Mo Tc Ru Rh Pd
Ag Cd In
Sn Sb
Te
Хе
S
LAZ
118.21
Ina
121ZE
127.
1260
112
th
81
comian
tant
55
56
57-70
71
72
73
74
15
76
77
78
79
B0
82
84
85
86
Cs Ba
Lu Hf Ta w Re
Os Ir Pt
Au Hg TI
Pb Bi Po At Rn
20438
s
108
112
87
88
89102
103
104
105
106
107
109
110
111
114
Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub
Uuq
CMER
erum
68
thi
69
Tavrerm
57
58
60
61
62
63
64
65
66
67
70
*Lanthanide series
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
1
Timan ra
142
T n n ern
97
**Actinide series
90
91
92
93
94
96
98
100
101
102
Ac Th Pa U Np
Pu Am Cm Bk Cf Es Fm Md No
Select one:
O a.
Zn, Cd, Hg
Ob.
Cu, Ag, Au
Oc.
Mn, Tc, Re
Od.
K, Rb, Cs
Sc, Y, Lu
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is the electron configuration of ground state arsenic atoms? ○ none of the above O [Ar]3s23d103p3 O [Ar]4s23d104p3 O [Ar]4s23d 104p4 O [Ar]4s13d104p4arrow_forwardIn the Periodic Table below, shade all the elements for which the neutral atom has an outer electron configuration of ms nd", where n and m are integers, and m=n+1. H He Li Be BCNOF Ne Na Mg Al Si PS CI Ar Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Zr Nb Mo Te Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn K Ca Sc Rb Sr Y Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cn Submit Assignment Continue O2021 McGraw Hil LLC. A Rights Reserved. Terms of UseI Privacy Center Accessibilityarrow_forwardELECTRONIC STRUCTURE AND CHEMICAL BONDING Identifying elements with a similar valence electron configuration In the Periodic Table below, shade all the elements for which the neutral atom has a valence electron configuration of ns'np, where stands for an integer. He B CNOF Ne Al Si P S Cl Ar Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn H Li Be Na Mg K Ca Sc Rb Sr Y Cs Ba La Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cn 0/3 Xarrow_forward
- In the Periodic Table below, shade all the elements for which the neutral atom has a valence electron configuration of ns²np5, where n stands for an integer. H Li Be Na Mg K Ca Sc Rb Sr Y Cs Ba La Fr Ra Ac B C Al Si P Ge As Ti V Cr Mn Fe Co Ni Cu Zn Ga Zr Nb Mo Tc Ru Rh Pd Ag Cd In Hf Ta W Re Os Ir Rf Db Sg Bh Hs Mt He OF Ne S Cl Ar Se Br Kr Sn Sb Te I Xe Pt Au Hg Tl Pb Bi Po At Rn Ds Rg Cn Harrow_forwardIn the Periodic Table below, shade all the elements for which the neutral atom has a valence electron configuration of ns“, where n stands for an integer. Не BCNOF Ne H Li Be Na Mg Al Si P S c1 Ar к Са Sc Ti V Cr Mn| Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Te Ru Rh Pd Ag Cd In Sn Sb Te I Xe Hf Ta W Re Os Ir Pt Au Hg T1 Pb Bi Po At Rn Rf Db Sg Bh Hs Mt Ds Rg Cn Cs Ba La Fr Ra Acarrow_forwardSelect all of the following ions that are paramagnetic in their ground state. Co3+ O Zn2+ Ti4+ Ag* Cr2+ 02-arrow_forward
- 2 5 In the Periodic Table below, shade all the elements for which the neutral atom has a valence electron configuration of n s np, where ʼn stands for an integer. H Li Be Na Mg K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Rb Sr Y Zr Nb Mo Tc Ru Rh Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cn He B CNO F Ne Al Si P S Cl Ar Br Kr Pd Ag Cd In Sn Sb Te I Xe Pb Bi Po At Rn X 5arrow_forwardIn the Periodic Table below, shade all the elements for which the neutral atom has a valence electron configuration of ns´np', where n stands for an integer. H Не B CNO Al Si PS CI Ar Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Te Ru Rh Pd Ag Cd In Sn Sb Te I Xe Cs Ba La Hf Ta w Re Os Ir Pt Au Hg TI Pb Bi Po At Rn Li Be F Ne Na Mg K Ca Sc Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg Cnarrow_forwardIn the Periodic Table below, shade all the elements for which the neutral atom has an outer electron configuration of ms²nd ¹, where n and m are integers, and m=n+1. H Li Be Na Mg K Ca Sc Rb Sr Y Cs Ba La Fr Ra Ac He O F Ne B C N Al Si P S Cl Ar Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Rf Db Sg Bh Hs Mt Ds Rg Cnarrow_forward
- the ground state of Mn+2 ion has how many unpaired electrons?arrow_forward13) Identify the transition metal ion and the number of electrons with the following electron configuration, [Ar]4s°3d7. A) cobalt (I) ion and 25 electrons B) cobalt (III) ion and 25 electrons C) cobalt (II) ion and 25 electrons 14) What is the electron configuration of a copper atom? A) [Ar]4s13d10 B) [Ar]4s°3d11 C) [Ar]4s?3d9arrow_forwardWhich of the following species is diamagnetic (not paramagnetic): Li, V, Ni, Zn, Fe2+ hon H He 10006 berm 10 Li Be BCN F Ne 5000 mages 12 age 18 11 13 14 15 16 17 Na Mg AI Si P S ci| Ar pots 27 kysk 36 19 20 21 22 23 24 25 29 31 32 33 34 35 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 38 39 40 41 42 43 44 45 46 47 48 49 51 52 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te Xe caian ad 82 as 85 56 57-70 71 72 73 14 75 76 77 78 79 81 83 84 Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn n 87 89-102 103 104 105 106 107 108 109 110 111 112 114 Fr Ra ** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub Uuq *Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb **Actinide series 90 91 92 93 $4 95 97 98 100 101 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Select one: a. V Ob. Fe2+ O. Zn o d. Li o e. Niarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY