
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
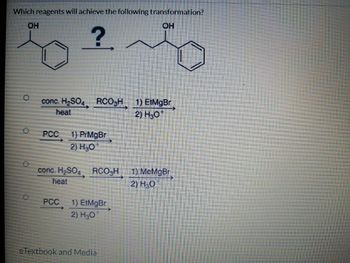
Transcribed Image Text:Which reagents will achieve the following transformation?
OH
?
O
O
PCC
conc. H₂SO4 RCO3H 1) EtMgBr
heat
2) H30
1) PrMgBr
2) H₂O
conc. H₂SO4 RCO₂H
heat
PCC _1) EtMgBr
2) H30
ملہ
eTextbook and Media
OH
MeMgBr
2) H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- EXAMPLE: Use the heat of solution data in Appendix I to determine the heat transferred per mole of entering solution into or out of (state which) a process in which 2 g mol of a 50 mol % solution of sulfuric acid at 25°C is mixed with water at 25°C to produce a solution at 25°C containing a mole ratio of 10 H₂O to 1 H₂SO4.arrow_forwardIf LiCl·3H2O(s) and H2O(l) are mixed isothermally at 25°C (298.15 K) to form a solution containing 15 mol of water for each mole of LiCl (LiCl(15H2O)), what is the heat effect per mole of the solution?arrow_forwardShow complete solutions. Round off final answers to 4 decimal places.arrow_forward
- 3) The molar heat of formation of NH&NO3 (s) is –367.54 kJ and those of N20 (g) and H20 (1) are 81.46 and -285.8 kJ respectively at 25 °C and 1 atmospheric pressure. Calculate AH, AS, and change in free energy of the reaction. NH,NO3(5) – N20o + H20marrow_forwardPleaseee help me with my hw. Tysm❤️ ACTIVITY: Answer completely 5 grams of an unknown compound was dissolved in 75 ml of water with an initial temperature of 22.5ᵒC contained in a coffee-cup calorimeter. Upon dissolution of the compound, the final temperature of the resulting solution is 33.2ᵒC. The calorimeter constant used is 200 J/ᵒC. What is the heat of the solution of the compound?arrow_forwardOnce the methanol has reached its boiling point, it must be vaporized. Themolar enthalpy of vaporization for methanol is 38 kJ mol-1 . How much heat must be added to vaporize 1.00 kg methanol?arrow_forward
- Given the following reaction: CO(g) + H₂O(g) CO2(g) + H2(g) Kc = 1.87 at 700 °C If a student heated 0.8 mol each of CO(g) and H2O(g) in a 5 L flask at 700 Celsius, solve for the concentration of each component when equilibrium is reached.arrow_forward9.20. Ammonia is oxidized with air to form nitric oxide in the first step of the production of nitric acid. Two principal reactions occur: 4 NH3 + 5 024 NO + 6 H₂O 2 NH3 + 2O₂ → N₂ + 3 H₂O A flowchart of the reactor follows. 100 mol NH3(g)/min 25°C, 8 bar 900 mol air/min 0.21 02 0.79 N₂ 150°C, 8 bar REACTOR Product gas: 700°C, 8 bar 90 mol NO/min 0000 150 mol H₂O(v)/min 716 mol N₂/min 69 mol 0₂/min ġ(kJ/min)arrow_forward8. The heat capacity of liquid water is 4.186 J/(g.oC) and that of isopropanol is 2.403 J/(g-OC). If they are equally mixed in a beaker, estimate the heat capacity of the mixture in J/(g-OCLarrow_forward
- PLEASE ANSWER THIS QUESTION ASAP!!!arrow_forwardall令只目 or O 25% 16:23 Calculate the standard Gibbs energy of the following reaction at 298 K, from the standard entropies and enthalpies of formation given in the table. CO(g) + CH;OH(1) → CH;COOH(1) Compound Sm°(J K'mol ) AH° (kJ mol') СО (9) 197.67 -110.53 СНЗОН (1) 126.8 -238.66 СНЗСООН () 159.8 -484.5arrow_forwardWrite and balance the chemical equation for the combustion reaction of propane (C3H₂) in air and determine the stoichiometric volume of air.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The