
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
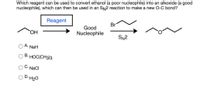
Transcribed Image Text:Which reagent can be used to convert ethanol (a poor nucleophile) into an alkoxide (a good
nucleophile), which can then be used in an SN2 reaction to make a new O-C bond?
Reagent
Br
Good
HO,
Nucleophile
SN2
А.
NaH
В.
HOC(CH3)3
· NaCl
D.H20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How does gold help to catalyze the reaction in the gold catalysis experiment? It deprotonates the methanol, making methanol a better nucleophile. оа. o b. It donates electron density to the alkyne, making the alkyne a better nucleophile. О с. It coordinates to the methanol, making methanol a better nucleophile. o d. It coordinates to the alkyne, making the alkyne a better electrophile.arrow_forwardFor the following Sy2 reaction, draw the organic and inorganic products of the reaction, and identify the nucleophile, substrate, and leaving group. Include wedge/dash bonds and H on a stereocenter N + Draw organic product Draw inorganic product Select the statement that properly identifies the nucleophile, substrate, and leaving group CN is the substrate, CI is the nucleophile, and CH3CH2CH2CH(CH3)Cl is the leaving group. Cr is the substrate, CH3CH2CH2CH(CH3)Cl is the nucleophile, and CN is the leaving group. CH3CH2CH2CH (CH3)CI is the substrate, CN is the nucleophile, and C is the leaving group.arrow_forwardDraw the major and minor product(s) of the following reaction? Br C NaOEt EtOH ?arrow_forward
- What is the first step of the mechanism when a strong nucleophile adds to a ketone? a) Protonation of the C=O oxygen b) Deprotonation of the C-H hydrogen c) Nucleophilic addition to the C=O carbon d) Nucleophilic addition to the C=O oxygenarrow_forwardhelp #2arrow_forwardChoose the reaction that involves a carbocation intermediate: Br REACTION A: NH2 CI REACTION B: OH CI Br2 REACTION C: FeBr3 Br REACTION D: OHarrow_forward
- Ethyl acetate can be prepared by an Sn2 reaction. Draw the alkylbromide and nucleophile used in the reaction. Remember to include formal charges and do not include counterions. H3C CH3 Draw the alkyl bromide. Draw the nucleophile. Select Draw Rings More More Erase Select Draw Rings Erase Br エ エarrow_forwardThis reaction is an example of conjugate addition of a nucleophile to an a,ẞ-unsaturated carbonyl. O CH3CH2CH2CH=CHCSCOA H₂O OH CH3CH2CH2CHCH2CSCOA Draw the two resonance structures of the enolate anion intermediate for this reaction. • Draw an R1 group in place of COA. The R group tool is located in the charges and lone pairs drop-down menu. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate resonance structures using the symbol from the drop-down menu. ->> 90-87 O + ? ChemDoodle >arrow_forwardWhich of the following is a substitution reaction? “人一X II. スーズ I. 00 OH "メー人 IV. III. Br 人ー人 SH 1) 1 2) 11 3) l1 4) IVarrow_forward
- How does the synthesis of these two reagents work?arrow_forwardIn the first step of a Wittig reaction, what will happen if you use chlorobenzene instead of benzyl chloride? Why a) the yield will increase, because chlorobenzene is a better nucleophile b) yield will be slightly decreased, because chlorobenzene is a poorer nucleophile c) no product will form, chlorobenzene cannot be converted to a phosphonium chloride salt d) no alkene product will form the phosphonium salt from chlorobenzene reacts with chloro benzene to form triphenyl magnesium chloride. e) no noticable change will be detected, benzyl chloride and chlorobenzene are essentially the same compoundsarrow_forward10. Consider the below reaction between the acetylide ion and methanol. Draw the conjugate acid that is formed as a product in this reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY