
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
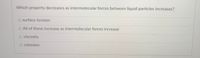
Transcribed Image Text:Which property decreases as intermolecular forces between liquid particles increases?
O surface tension
O All of these increase as intermolecular forces increase
o viscosity
O cohesion
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Which evaporates more quickly: 55 mL of water in a beaker witha diameter of 4.5 cm or 55 mL of water in a dish with a diameterof 12 cm? Is the vapor pressure of the water different in the twocontainers? Explain.arrow_forwardBased on your knowledge of intermolecular forces, a container of liquid SOBr, has a higher vapor pressure than a container of liquid Br2. O True O Falsearrow_forwardFor the liquids, CH,F,, CH,Cl, and CH,I, at the same temperature, rank them in order of: a. increasing strengths of intermolecular forces b. increasing viscosity foin we c. increasing surface tension d. increasing vapor pressuresarrow_forward
- Which of the following physical properties depend on intermolecular forces . | surface tension Il vapor pressure III melting point IV boiling point viscosity V I and IV II, III, and IV I and V I, II, and IV They all depend on intermolecular forcesarrow_forwardExplain the molecules in a solid are kept at their positions by the large springlike intermolecular forces.arrow_forwardWhich of the following statements is FALSE? Vapor pressure increases with increasing temperature Assuming similar molar mass, molecules with hydrogen bonding are more volatile than compounds with dipole-dipole forces Vapor pressure increases with decreasing strength of intermolecular forces.arrow_forward
- In a liquid, the tendency of liquids to minimize their surface area is called O capillary action O dipole - dipole forces O Surface tension O viscosity OH- bondingarrow_forwardWhat are the strongest intermolecular forces in SF,? ) covalent bonds )London forces O dipole-dipole forces O hydrogen bonds ion-dipole forcesarrow_forwardSolids have a greater strength of attraction between its molecules than liquids True Falsearrow_forward
- The types of intermolecular forces in a substance are identical whether it is a solid, a liquid,or a gas. Why then does a substance change phase from a gas to a liquid or to a solid?Explain using the intermolecular forces present in a water molecule. (you might want torefer to unit 2)arrow_forward4. What type of intermolecular attractions can occur for each molecule? (intermolecular forces between molecules of the same kind). H CH;NH2 H- -N -H H %23 H H H- H- C2H6 SİH2O H.arrow_forwardThe molecules in a sample of solid SO, are attracted to cach other by a combination of O London forces and H-bonding OH-bonding and ionic bonding O just London forces O London forces and dipole-dipole interactions O none of thesearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY