
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
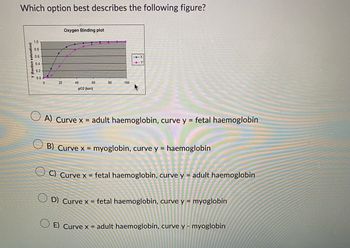
Transcribed Image Text:Which option best describes the following figure?
Y (fraction saturation)
1.0
0.8
0.6
0.4
0.2
0.0
0
20
Oxygen Binding plot
40
60
p02 (torr)
80
100
A) Curve x = adult haemoglobin, curve y = fetal haemoglobin
B) Curve x = myoglobin, curve y = haemoglobin
C) Curve x = fetal haemoglobin, curve y = adult haemoglobin
D) Curve x = fetal haemoglobin, curve y = myoglobin
E) Curve x = adult haemoglobin, curve y - myoglobin
Expert Solution
arrow_forward
Step 1
Hemoglobin and myoglobin are heme group proteins that can bind to oxygen.
While hemoglobin is found all over the body and myoglobin is found only in muscle tissues.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 7L.4.2arrow_forwardTalk about the factors that can move the oxygen-hemoglobin dissociation curve TO THE RIGHT. What factors move the oxygen-hemoglobin dissociation curve TO THE LEFT? What does this mean, exactly?arrow_forwardA 50-year-old female patient has an FEV1 of 1.8 liters and an FVC of 3.2 liters. What is the patient's FEV1/FVC ratio, and what does it suggest about the patient's lung function? 0.39, severe obstruction O 0.56, moderate obstruction 0.69, mild obstruction O 0.78, normal lung functionarrow_forward
- Which of the following is the concentration of hemoglobin-bound oxygen in the blood when the heme is fully saturated? 8500 uM 8630 uM 2200 uM 8800 uMarrow_forwardBelow are multiple oxygen binding affinity curves for hemoglobin. The affinity curve for normal hemoglobin in blood is represented by curve "D," at a pH of 7.2 and at a concentration of 5mM BPG and 26 mM CO2. O₂ saturation (%) 100 Answer 1: B Answer 2: [Select] Answer 3: B Answer 4: 80 A 60 1. How would changes to acidity, BPG concentration and CO₂ levels affect the binding affinity curve and p50? lower than Answer 5: E 40 1. BPG concentration is decreased to 3mM: The binding affinity curve would look like B. The p50 would be lower than normal (D). 2. pH is increased to 7.6: The binding affinity curve would look like B. The p50 would be [Select] normal (D). 20 3. CO₂ concentration is increased to 30 mM: The binding affinity curve would look like. [Select] The p50 would be Answer 6: 0 normal (D). II. The hemoglobin has been treated with a denaturing solution which disrupts quaternary structure only, and has a binding affinity resembling myoglobin. The binding affinity curve would look…arrow_forwardSuppose you visit the Dalai Lama in Dharamsala, India (elevation 1460 m),and you begin to ponder the “big questions,” such as “What is the fractionalsaturation of the Dalai Lama’s hemoglobin?”(a) Assuming the Dalai Lama’s hemoglobin has a Hill coefficient = 3.2,and a P50 = 31 mm Hg, calculate the change in fractional O2 saturationof his hemoglobin going from his lungs (where PO2 = 85 mm Hg) to hiscapillaries (where PO2 = 25 mm Hg).(b) Why do you suppose the Dalai Lama’s hemoglobin has a P50 higher thannormal (where “normal” = 27 mm Hg)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON