
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
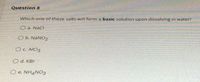
Transcribed Image Text:Question 8
Which one of these salts will form a basic solution upon dissolving in water?
O a. NaCl
O b. NaNO2
OC. AICI3
O d. KBr
O e. NH4NO3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 29 The pH of an aqueous solution at 25°℃ WAS found to be 7.30. S S The hydrodium ion concentration is The hydroxide ion concentration is in stan M. m.arrow_forwardWhat is the pH of a solution made by mixing 40.0 mL of 0.20 M HCl with 40.0 mL of 0.10 M KOH? a. 1.30 b. 0.20 c. 0.05 d. 0.30arrow_forwardWhich one of the following salts forms a basic solution when dissolved in water (25°C)? a NaNO3 b) KI c) Li2SO4 d) KBr e) KCI f) KFarrow_forward
- Please answer # 82arrow_forwardIdentify the conjugate acid and conjugate base of the following acids and bases. Then, write the equation for each reaction. a. HCO2H and OH b. HSO4 and PO4³- c. HCN and NH3 d. HNO2 and H₂O e. NCIO3 and OH-arrow_forward2. For each of the following salts, determine the pH of a 2.50M solution of the salt. You will need the Ka and Kb tables to complete this problem. a. NaBro wimm b. CH3NH3CI c. NaClO₂ d. C6H5OK e. CsNO3 f. C6H5NH3Brarrow_forward
- One aqueous solution of a strong acid has pH = 1.20, another pH = 1.80. If equal volumes of these two solutions are combined, what is the pH of the resulting solution? A. 1.35 B. 1.50 C. 1.40 D. 1.55 E. 1.10arrow_forward3. Which of the following should produce the lowest pH when dissolved in water (assuming equal concentrations dissolved)? A. HCIO B. LiOH C. HC2H3O2 D. HOCNarrow_forward3. Which of the following should produce the lowest pH when dissoived in water (assuming equal concentrations dissolved)? A. HCIO B. LIOH C. HC;H,O, D. HОCNarrow_forward
- 9. Classify solutions in the previous problem as acidic, basic, or neutral a. [H+] = 1 x 10^-5 M b. [H+] = 4.4 x 10^-11 M c. [OH-] = 2.2 x 10^-7 M d. pOH = 1.4 $ 4 R F % 5 T G A 6 MacBook Pro Y H & 7 U * 00 8 9arrow_forwardPredict whether aqueous solutions of the following compounds are acidic, basic, or neutral. compound solution is...? acidic NH₂Cl basic neutral acidic AI(CIO4)3 basic neutral acidic NaNO3 basic neutral X 5 ?arrow_forwardThe following 3 substances are ionic. Indicate what effect (increase, decrease, no change) each will have on pH when added to water. a. HCl b. NaOHc. NaClarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY