
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
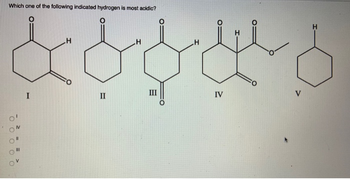
Transcribed Image Text:Which one of the following indicated hydrogen is most acidic?
O
#1
MI
I
H
II
H
III
H
IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the correct answer and why?arrow_forwardWhich of the following will be the strongest base? Br థరూరలో B CF3 OH Darrow_forwardactice ! 1 201 FI Q A N 3.4 Brønsted-Lowry Acidity: Factors Affecting the Stability of Anions Considering the hydrogen atoms highlighted in red in the two compounds shown below, which is more acidic and why? 2 A Bos Save for Later W -H OA is the stronger acid because its conjugate base is stabilized by resonance. OB is the stronger acid because its conjugate base is stabilized by resonance. S OA is the stronger acid because the carbon atom bearing the highlighted proton is sp² hybridized. OB is the stronger, acid because the carbon atom bearing the highlighted proton is sp³ hybridized. X # VS. 3 80 F3 E D B $ 4 C 888 F4 R H F % 5 V T G ^ 6 MacBook Air B F6 Y & 7 H F7 U N * 8 J DII FB 1 ( - o M 9 K DD FO O ) Submit Answer O P E FI { [ Aarrow_forward
- Which acid in each of the following pairs is stronger?arrow_forwardWhich is the most acidic hydrogen in this compound? Ha Hb Ha - C-C-N_: IK На Hb 0 Ha O Hb O Hc O No answer text provided. Hcarrow_forwardPlease provide only typed answer solution no handwritten solution needed allowed which one is more acidic between each moleculearrow_forward
- Arrange the following compounds based on their increasing acidity. Write 1 for weakest acid, 2 for the next, then 3, then 4 and 5 for strongest acid,arrow_forwardOrder the following carboxylic acids from the least to most acidic Most Acidic T 2 3 **** or F OH OH OHarrow_forwardWhich compound is the most acidic? Which compound is the least acidic? Rationalize acidity based on molecular structure.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY