
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
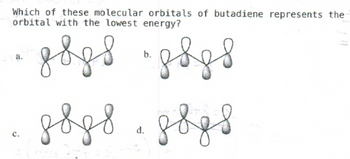
Transcribed Image Text:Which of these molecular orbitals of butadiene represents the
orbital with the lowest energy?
двдв "двдв
двдв двув
a.
с.
d.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- . The pyridine molecule (C3H;N) is obtained by replacing one C-H group in benzene with a nitrogen atom. Because nitrogen is more electronegative than the C-H group, orbitals with electron density on nitrogen are lower in energy. How do you expect the MOs and energy levels of pyridine to differ from those of benzene?arrow_forwardWhat is the molecular orbital structures of 1,4-pentadiene? What about 1,4-pentadiene radical?arrow_forwardHow many nodes are present in the LUMO (lowest unoccupied molecular orbital) of 1,3-butadiene? O 4 3. O 1arrow_forward
- How many nodes are there in each of the MOs of 1,3-butadiene?arrow_forwardHow many π-MOs are formed from the linear combination of AOs contributing to the π-bonding in 1,3-pentadiene? Explainarrow_forwardWrite a bond-line structure for the following compounds. Present correct geometry for sp3, sp2, andsp hybridized carbon atoms. b.-OOCCHCHCCCH2COOH, The molecule is negatively charged, the double bond has transgeometryarrow_forward
- Anthracene is a yellow, crystalline solid found in coal tar. Complete the structure for anthracene, CH0, by adding bonds and hydrogen atoms as necessary. Select Draw Rings More Erase C H What type of hybrid orbitals are utilized by carbon in anthracene? Question Source: McQuarrie, Rock, And Gallogly 4e General Chemsitry PublisherG University Science Booksarrow_forwardIf you could please help me figure this one out. thank you!arrow_forwardDraw in the orbitals for the oxygen's lone pairsWhich structure allows the oxygen lone pair to be in an orbital that is aligned with the pi bond (conjugated)?Draw in the P orbitalsCircle the atoms in each structure that are conjugatedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning