
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:Which of the structures have a major peak above 3000 cm1? (Select all that apply).
HO,
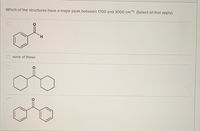
Transcribed Image Text:Which of the structures have a major peak between 1700 and 3000 cm1? (Select all that apply).
of
O none of these
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Page 9 Problem #2) An absorption spectrum includes the transition between n(hi) = 17 and n(lo) = 5. a) Calculate the wavelength in nm; b) Calculate delta E in kJ/mol; c) Draw the energy level diagram.arrow_forwarddelta w = 0 when: (a) delta t=0 (b) delta P = 0 (c) delta V = 0 (d) delta P>0 (e) delta T>0 (f) delta P>0arrow_forwardANswer thsi using particle in a box model for conjugated pi systems. A model has 12 double bonds with carbon backbone of 2.4 x 10^-9 m. what js the energy and color of light absorbed to excite an electron to next lowest energy level? and with 10 double bonds?arrow_forward
- Why is it incorrect to speak of a molecule of solid NaCl?arrow_forwardDumb question, just starting to learn about mass spectometry. I know we have to memorize the common radical fragments, M-15, M-29, etc, but lloking at the example, those numbers dont crrespond to the absorbance or the m/z, so for example, M-29. The absorbance for M-29 is at 100 , while the m/z is at 43. How is it designated to be M-29?arrow_forwardChallenge Problem: The following are three sets of data for the atomic mass of antimony from the work of Willard and McAlpine': Set 1 Set 2 Set 3 121.771 121.784 121.752 121.787 121.758 121.784 121.803 121.765 121.765 121.781 121.794 (a) Determine the mean and the standard deviation for each data set. (b) Determine the 95% confidence interval for each data set. (c) Determine whether the 121.803 value in the first data set is an outlier for that set at the 95% confi- dence level. (d) Use the t test to determine whether the mean of data set 3 is identical to that of data set 1 at the 95% confidence level. (e) The means of all three data sets are to be compared by ANOVA. State the null hypothesis. Determine whether the means differ at the 95% confidence level. (f) Pool the data and determine the overall mean and the pooled standard deviation. (g) Compare the overall mean of the 11l data points to the currently accepted value. Report the absolute error and the relative error in percent…arrow_forward
- prelab 4) what is beer's law in terms of concentration and absorbance of the absorbing species?arrow_forwardProject 2: Food Dye Spectroscopy What method will you use to assess the absorbance-concentration relationship? (The most obvious method is to see if there is a linear relationship between the absorbance and the concentration. What methods do you know that might reveal such a linear relationship?)arrow_forwardChamistryarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax