
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Could you help me with this question? I don't know where to start. All the information has been provided.
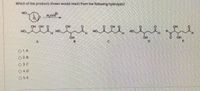
Transcribed Image Text:Which of the products shown would result from the following hydrolysis?
но.
H,O/H
OH OH O
OH
OH O
OH
HO.
HO
H.
но.
HO
OH
B.
OH
E
O 1.A
O 2. B
O 3. C
O4. D
O 5. E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Even though cement and bread are not substances, they can help you to understand these concepts: Whatever you do to volume affects mass the same way. Whatever you do to mass affects volume the same way. If you have half as much of a material, you would expect the volume of the material to also be half as much. If you have twice as much of the material, you would expect its volume to also be twice as much. Substances, which you know are always the same throughout, will follow this rule. This relationship between mass and volume will stay the same for any substance, no matter how much of that substance you can sample and measure in the classroom. Why is this principle about the relationship between mass and volume always true of substances, but not always true of mixtures?arrow_forwardNow it's time to create, analyze and interpret a Chemistry-based graph! Let's do this in steps. Data Measurement number Temperature (°C) Solubility (g NaCl/100 g water) 1 0.00 35.2 2 20.0 36.1 3 40.0 37.0 4 60.0 37.7 5 80.0 38.4 100.0 39.0 Let's assume that you just finished your first research laboratory experiment and you collected the data listed above. Pay close attention to the titles. Based on the titles listed answer and complete the following: a. What would be the title of the graph? b. What set of data would you place on the y-axis? This is your dependent variable-measurable variable whose value depends on the independent variable. c. What set of data would you place on the x-axis? This is your independent variable-measurable variable of which when changed, the value of the dependent variable changes. d. Based on the data listed above, use the following site to make a Line Graph of the data listed above and turn it in here. e. Give a detailed CER writing response to the…arrow_forward) A 1 gallon bottle of a certain brand of bleach costs $1.49. Determine the cost to buy enough bleach to supply 100.0 grams of active ingredient (NaOCl), assuming 6.25% active ingredient by mass and a density of 1.05 g/ml.arrow_forward
- Dimensional Analysis is a way of doing numerical "book-keeping" when converting quantities or performing calculations. • When converting quantities from one unit to another, conversion factors are used. Solving with Dimensional Analysis and Multiple Units: If I am in Canada where the price of gas is $1.022 USD·L1, how much will it cost me to fill up my gas tank if I travelled 125 km? • Let's also assume that my car gets an average of 30.0 miles/gallon.arrow_forwardEthanol has a density of 0.789 g cm‐3. Calculate the volume in mL that must be poured into a measuring cylinder to give 19.8 g of ethanol. Note 1 mL = 1 cm3.arrow_forwardA hypothetical metal has a theoretical density of 11.47 g/cm3. This metal adopts a cubic crystal structure with an edge length of 0.387 nm and atomic radius of 0.137 nm. Find the atomic weight of this metal in g/mol. NA = 6.022 x 1023 atoms/mol. Express your answer in two decimal places only. Do not put the units.arrow_forward
- Blood alcohol content is a measure of alcohol in the blood as a percentage. It is calculated in grams per 100 mL of blood, so a BAC of 0.08 means your blood is 0.08% alcohol by volume. what is the total mass of alcohol (in grams) that is present for the same adult male whose total blood volume is 5.6L?arrow_forwardA chemistry student weighs out 0.117 g of hypobromous acid (HBrO) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1600 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Be sure your answer has the correct number of significant digits. mL 0 x10 Xarrow_forwardWhat is calibration and why is it essential in relation to food analysis? Provide examples.arrow_forward
- Suppose some measurements are made on two different homogeneous stones to find out if they are made of the same kind of rock. The mass and volume measurements are listed below. Are the two stones the same type of rock? Why or why not? Show all calculations. Mass Volume Calculations Stone 1 58.0 g 20.0 cm Stone 2 50.1 g 15.0 cm3 21/common/assets/pdfjs/1.0.0.30/web/viewer.ht...ndered-pdf&fullscreen=Dd21-fileviewer-rendered-pdf-dialog&height=746#0arrow_forward15arrow_forward4. Gasoline can be reasonably approximated as C9H20 with a density of 0.718 g/mL. Diesel can be reasonable approximated as C14H30 with a density of 0.763 g/mL. (Both gasoline and diesel are complicated mixtures. These formulas are something like weighted averages of the formulas of the different components, where the weighting is based on various quantifiable behaviors. That is, using these artificial formulas for gasoline and diesel allows one to calculate average properties that match what petroleum engineers might measure.) The enthalpy change from burning one mole of gasoline is -6160 kJ. The enthalpy change from burning one mole of diesel is -7940 kJ. Determine which fuel generates the biggest enthalpy change per gram burned, and which generates the biggest enthalpy change per mL burned.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY