
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:10:13
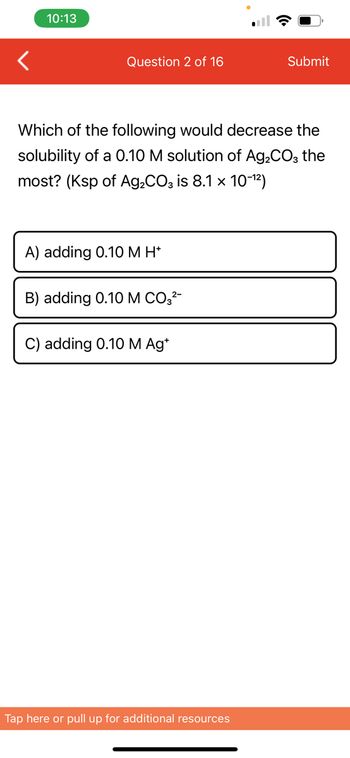
Question 2 of 16
Which of the following would decrease the
solubility of a 0.10 M solution of Ag₂CO3 the
most? (Ksp of Ag₂CO3 is 8.1 × 10-¹²)
A) adding 0.10 M H*
B) adding 0.10 M CO3²-
C) adding 0.10 M Ag*
Submit
Tap here or pull up for additional resources
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the solubility (in grams per liter) of magnesium hydroxide (Molar mass = 58.32 g/mol, Ksp = 6.0 x 10-12) in a) pure water b) 0.041 M Ba(OH)2 c) 0.0050 M MgCl2arrow_forwardWhat is the molar solubility of strontium carbonate in a solution that contains 0.19-M SrCl2?Ksp(SrCO3) = 9.3 × 10-10 mol/L=arrow_forwardFor which of the following saturated solutions will a decrease in pH decrease the solubility of the solid? A) CH₂NH₂Br B) HCOONa C) LICIO D) RbNO, 2 E) A and Darrow_forward
- 3.a) Iron (II) hydroxide, Fe(OH), has a K, value equal to 4.87×10-17. What is the molar solubility (S) of Iron (II) hydroxide. 3.b) What are the concentrations of the [Fe**] and [OH¯].arrow_forwardCalculate the molar solubility of Mg(OH) 2 in a solution with pH = 12.20. (K sp (Mg(OH)₂ = 1.2 x 10¹¹) A) 7.6 x 10⁻¹⁰ M B) 3.5 x 10⁻⁶ M C) 1.4 x 10⁻⁴ M D) 4.8 x 10 ⁻⁸M E) 2.4 x 10 ⁻⁶ Marrow_forwarddhd See Periodic Table See Hint The solubility of magnesium hydroxide. Mg(OH)2, in water is 9.0x 10g/100 mL What volume of 6.50×10 MHNO, is required to neutralize 1.00L of saturated Mg(OH), solution? ml mlarrow_forward
- For which of the following saturated solutions will an increase in pH increase the solubility of the solid? A) Na₂CO3 B) Sr(CIO4)2 C) NH₂Br D) Na₂CrO4 E) None of these.arrow_forwardAt a certain temperature, the solubility of strontium arsenate, Sr3(AsO4)2, is 0.0650 g/L. What is the Ksp of this salt at this temperaturearrow_forwardNonearrow_forward
- 19) What is the molar solubility of AgCl in 0. 30 M NH3? Ksp for AgCl is 1.8 x 10-10 and Kf for Ag(NH3)2+ is 1.7 × 107. A) 1.3 x 10-5 M B) 1.6 x 10-2 M C) 1.7 x 10-2 M D) 5.5 × 10-2 Marrow_forwardQuestion 6arrow_forwardWhich salt(s) would you expect to have a higher solubility at lower pH? List all that apply. AgCN AgCl BaF2 Mg(OH)2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY