
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
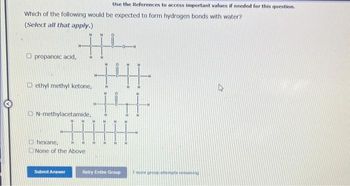
Transcribed Image Text:Use the References to access important values if needed for this question.
Which of the following would be expected to form hydrogen bonds with water?
(Select all that apply.)
propanoic acid, H
ethyl methyl ketone,
N-methylacetamide,
hexane,
None of the Above
Submit Answer
14
H
Retry Entire Group
7 more group attempts remaining
27
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- Type of non-bonding interaction which can exist in methane or CH4 hydrogen bond Induced dipole permanent dipolearrow_forwardWhich of the following shows the correct hydrogen bonding between the below compound and water (H2O)? b)arrow_forwardWhich of the following compounds can form hydrogen bonds with water molecules? Do CH₂-CH₂-C-H all of them. 요 CH3-C-CH3arrow_forward
- Rank the forces overall of methane, methanol and water (IMF: dispersion, dipole-dipole, hydrogen-bonding)arrow_forwardBased on bond polarity and VSEPR shape, for which of the following covalent compounds are dipole-dipole attractions the strongest intermolecular force? Choose ALL that apply. a) Ammonia, NH3 b) Benzene, C6H6 c) Carbon Monoxide, CO d) Nitrogen Tribromide, NBr3 e) Sulphur Hexafluoride, SF6 f) Sulphur Dioxide, SO2arrow_forward2:35 Question 8 of 11 Submit Which one of the following would have the largest dispersion forces? A) CH;CH,SH B) CH;NH2 C) CH, D) CH;CH3 Tap here or pull up for additional resourcesarrow_forward
- which option is correct The boiling point of water at sea level is 100 °C. At higher altitudes, the boiling point of water will be Group of answer choices higher, because there are fewer water molecules in the air. higher, because the altitude is greater. the same, because water always boils at 100 °C. lower, because temperatures are lower. lower, because the atmospheric pressure is lower.arrow_forwardWhich of the following statements are correct? More than one answer is possible. (Select all that apply.) O LiF will have a higher vapor pressure at 25 °C than H2S. NH3 is more soluble in CCI4 than in water. O NH3 will have a higher vapor pressure at 25 °C than Beo. O As the polarity of a covalent compound increases, its solubility in water increases. U NH3 will have a lower vapor pressure at -50 °C than NO.arrow_forwardQuestion 16 Draw the Lewis structure for a bromine molecule, Br2. When the substance of bromine melts, what interaction(s) are overcome? Select all that apply. OMetallic bonds O Dipole-dipole interactions OHydrogen bonding interactions Covalent bonds London Dispersion forcesarrow_forward
- If a solid line represents a covalent bond and a dotted line represents intermolecular attraction, which of the choices shows a hydrogen bond? (select all that apply) A. H2O⋅⋅⋅⋅⋅⋅H−CH3 B. H−H C. H3N⋅⋅⋅⋅⋅⋅H−O−H D. H4C⋅⋅⋅⋅⋅⋅H−Farrow_forwardplease anwer themarrow_forwardwhich of the following is true for liquid water that is boling? as it boils liquid attractive forces between liquid water molecules are broken allowing gasesous water molecules to excape as it boils liquid water breaks down into hydrogen gas and oxygen gas as it boils liquid water molecules realease two hydrogen ions as oxygen gas escapes. as it boils air trapped between water moleculs forms bubbles that rise to the surface and excapearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY