
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
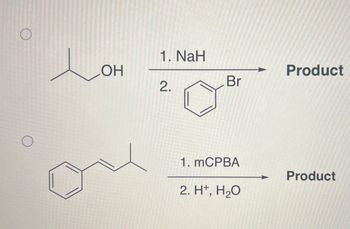
Transcribed Image Text:O
O
OH
1. NaH
2.
Br
1. mCPBA
2. H+, H₂O
Product
- Product
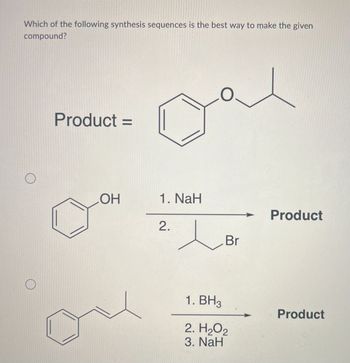
Transcribed Image Text:Which of the following synthesis sequences is the best way to make the given
compound?
O
Product =
=
OH
Dal
1. NaH
2.
Br
1. BH3
2. H₂O2
3. NaH
Product
Product
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- Predict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced and contains state symbols after every reactant and product. HCIO (aq) + H2O(l) → ローロ Х 5 2 00 Ararrow_forwardWhich of the following represents a combustion reaction? A. 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g) B. LiOH(aq) + HNO3(aq) → LiNO3(aq) + H2O(l) C. N2(g) + 3H2(g) → 2NH3(g) D. 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l) E. 2Al(s) + 3H2SO4(aq) → Al2(SO4)3(aq) + 3H2(g)arrow_forwardA flame test was carried out on solid sodium carbonate. The flame colour will be A Red B Lilac C Green D Yellow Oarrow_forward
- Balance each chemical equation MgS(s) + HCl(aq) ----> MgCl2(aq) + H2S(g)arrow_forwardH2. Consider the following reaction: CH4 + 2O2 --> 2H2O + CO2 How many moles of water can be formed from 3.7 moles of CH4 and 3.0 moles of O2? Explain with detailsarrow_forwardConsider the synthesis of ammonia: N2 (g) +3H, (g) 2NH3 (g)arrow_forward
- Use the following information to answer this question. 2Al) + 3CuCl2(aq)→ 2AICI3(aq) + 3Cu(s) (word equation: aluminum plus three copper(Il) chloride produce two aluminum chloride and three copper) Which of the following best describes what is occurring during this reaction? Select one: O a. Aluminum replaces chlorine in the compound. O b. Aluminum replaces copper in the compound. O c. Copper replaces aluminum in the compound. O d. Chlorine replaces copper in the compound. Smoke Signal.pdf EFiles (1).zip Chap6 SF10 (1).pdfarrow_forwardWhat type of reaction is this? Pb + H3PO4 --> H2 + Pb3(POa)2 What type of reaction is this? NaBr + Ca(OH)2 --> CaBr2 + NaOHarrow_forwardWhat mass of CO2 can be produced by the complete combustion of 2.00 kg of octane, C8H18, a major component of gasoline, in 8.00 kg of oxygen?arrow_forward
- One way to analyze for the amount of phosphorus in a rock is to precipitate the phosphorus as MgNH PO4, which is then heated to tum it into Mg,P2O7. What mass of Mg2P207 will be obtained from a 2.102 g sample of a rock that contains 86.52% Ca3(PO4)2 (and no other source of phosphorus) if this analysis is done? Hint: you will not be able to write an equation for this problem. Please do not include units in your answer. MAR MacBook Air 2.arrow_forwardWhat mass of water is produced from the complete combustion of 2.40×10−3 g of methane?arrow_forwardHow many grams of NaCl should be produced if you start with 1.0 g of sodium bicarbonate. This conversion will require three conversion factors. Start with 1.0 g NaHCO3. Then use a conversion factor that converts that to moles. Then use the balanced equation to convert from moles of NaHCO3 to moles of NaCl. Then use a conversion factor to convert to grams.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY