
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
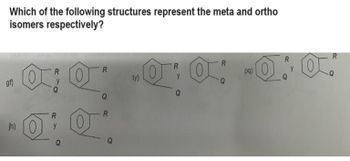
Transcribed Image Text:Which of the following structures represent the meta and ortho
isomers respectively?
gf)
R
y
jh) O 0
R
y
R
Q
R
ty)
R
·0:0:-0.0.
pq)
R
y
R
Q
R
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the line-angle (skeletal) notation of the saturated (maximum possible number of hydrogen atoms attached to the carbon atoms) hydrocarbon chain that is included in the R group. о CH₂O-C-R о CH₂OH 0 R-C-ONa+ о = CH-O-C-R 3 NaOH CH-OH R-C-O Na о 0 = CH₂O-C-R CH₂OH R-C-ONa+arrow_forwardQB=0&inProgress=0 Which of the following is the correct structure for (S)-1,1,3-trimethylcyclohexanearrow_forward0 X The functional groups of the central carbon are COO-, NH3+, and H True false 14 3 Traducer hparrow_forward
- Determine whether the two structures in the following pairs represent diastereomers, enantiomers, structural (constitutional) isomers, identical molecules, or are unrelated. OH H. C=c=c;' Br Br CC=c=c; Но- Br and H. Br a Br Br 23 c abubonq I2 be Sa H CI 01 CH3 CI, H and Br Co,H and Br H3C HO,C ci H bns Sa H CI Br H. H3C- H- H3C- Br H,C CH Br and and H- -CH3 CH3 Br CH3 CH3arrow_forwardс ← + C Q A N app.aktiv.com How many hydrogen atoms are in the skeletal structure of the alkane shown below? F1 @ 2 W F2 # 3 E D 80 $ 4 XI C JAN 25 A R LL. F % 5 V 0 F5 T ^ 6 Question 24 of 26 G e Y B & 7 H U DII 8 N FB zoom DD F9 + K Marrow_forwardWhich compound is a structural isomer of the following organic compound? O a CH3 OH Ob. H.C CH3 Od. H3C Oe. e here to search A F2 F3 F4 ES F6 F7 F8 F9 F10 F11 F12 #3 2$ 2 a 3 £ & T Y S D F H J KL 立arrow_forward
- 6.arrow_forwardGive the name for the structure in the image shown: CH,CH, CH,CH, CH3CCH,CH CH3 CH3 O 2,4-dimethyl-2,4-diethylbutane O 3,5,5-trimethylheptane O 1,3-dimethyl-1,3-diethylbutane O 3,3,5-trimethylheptanearrow_forwardName the following compounds: a Br CH-C=C-C-CHる CH3 d. Br e.arrow_forward
- The following pair of molecules are best described as x} O unrelated compounds E/Z isomers constitutional isomers cis/trans isomers the same compoundarrow_forwardIdentify the carbon atom that should be designated as #1 when naming this compound systematically. CH3 Which carbon is C1? A E A -CH2CH3 H3C D E Give the systematic name for the compound. Spelling and punctuation count! Name:arrow_forwardGive the name for each of the following compounds: Structural Formula Name Cнз .CH2 CH сH-ҫ—сHҙ CHz CH2 CHз CHз Cна -CH CH2- -CH снз CH2 CH2 CH-CH2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY