
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
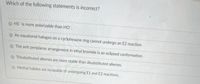
Transcribed Image Text:Which of the following statements is incorrect?
O HS is more polarizable than HO.
An equatorial halogen on a cyclohexane ring cannot undergo an E2 reaction.
The anti periplanar arrangement in ethyl bromide is an eclipsed conformation.
O Trisubstituted alkenes are more stable than disubstituted alkenes.
Methyl halides are incapable of undergoing E1 and E2 reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1.Draw a structural formula for the more stable carbocation intermediate formed in the reaction shown. 2.Draw a structural formula for the major organic product of the reaction shown.arrow_forwardWrite the structure of the compound that will be produced in the following reaction? CH3 –C ≡ C–CH2– CH2 – CH3 + 2HBr→arrow_forwardGive the systematic (IUPAC) names for these molecules. Boononon cnolonongron. НаСНз CHОССH2СH2СНCHЗ CH3 phenyl propanoate |4-methyl pentane methanoat Incorrect. You mixed up the acyl and alkoxy portions of the molecule. Name the alkoxy part first, followed by the acyl part.arrow_forward
- Draw the structure of the expected organic product(s) formed in the following reactions including correct stereochemistry. If the product is racemic write both isomers or write racemic. Assume all reagents listed are present in excess unless otherwise noted. If no reaction occurs, state 'No Reaction'arrow_forwardDraw a structural formula for the major product of the reaction shown. Br2 =CHCH2CH3 H20 • Show product stereochemistry IF the reactant alkene has both carbons of the double bond within a ring. • Do not show stereochemistry in other cases. • If the reaction produces a racemic mixture, just draw one stereoisomer. aste ChemDoodle Previous Next Save and Exi tv MacBook Air DD 80 888 F11 F12 F9 F10 F7 F8 F5 F6 F3 F4 & ( ) #3 $ % %3D 3 4 6 7 8 9. { } P E Y U D G H J K F > .. .. Rarrow_forwardWhich of the following compounds produces four monobrominated products if reacted with Br2 in the presence of uv light (Note: stereochemical products are excluded). 3,3-Dimethylhexane 2,2-Dimethylpentane 3,3-Dimethylpentane 2,2-Dimethylpropanearrow_forward
- Which is not an alkene formed when 2-chloro-2-methylbutane is dehydrohalogenated with NaOCH2CH3 (sodium ethoxide)? O 2-methyl-but-2-ene O 1-methylcyclobut-1-ene 2-methyl-but-1-enearrow_forwardDraw a structural formula for the product formed upon hydroboration/oxidation of the alkene below. CH;CH2CH,CH2 C=CHCH3 CH;CH,CH,CH2arrow_forwardWhich of the following statements is incorrect? The carbon atom in CH3* is sp2-hybridized. Vinyl halides do not undergo E2 reactions. Alkyl halides have higher melting points than alkanes of comparable size. Cations are well-solvated in both protic and aprotic polar solvents.arrow_forward
- Draw the major elimination and substitution products formed in this reaction. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, where applicable. Ignore any inorganic byproducts. 1 H₂O CI Q heatarrow_forwardDraw the structure of the expected organic product(s) formed in the following reactions including correct stereochemistry. If the product is racemic write both isomers or write racemic. Assume all reagents listed are present in excess unless otherwise noted. If no reaction occurs, state 'No Reaction'arrow_forwardNAME and DRAW the STRUCTURE of ALL the alkenes which could undergo catalytic hydrogenation at 900°C to form methylcyclopentane. Circle the alkene with the HIGHEST stability and X the alkene with the HIGHEST heat of hydrogenation. Give reasons for your choice.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY