
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
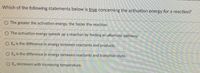
Transcribed Image Text:Which of the following statements below is true concerning the activation energy for a reaction?
O The greater the activation energy, the faster the reaction.
The activation energy speeds up a reaction by finding an alternate pathway.
E, is the difference in energy between reactants and products.
O E, is the difference in energy between reactants and transition state.
Ea decreases with increasing temperature.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- %23 Consider a hypothetical chemical reaction: A+B → C+D (In this equation A, B, C and D stand for some unknown chemical formulas.) Here is an energy diagram for the reaction: 400 300 energy 200 (k]/mol) C + D 100 - A + B reaction coordinate Use the energy diagram to answer these questions. What is the heat of reaction? O KJ/mol O Exothermic Is the reaction exothermic or endothermic? O Endothermic O Neither 00 O Yes, it's kJ/mol Can you determine the activation energy? Explanation Check O 2021 McGraw-Hill Education. AI Rightseserve Terms of MacBook Air 30 88 esc F10 F7 F6 F9 F1 F2 F3 F5 @ #3 24 % & 8. 6.arrow_forwardThe catalyst influences the rate of reaction in which ways? nothing happens O Decreases it O Increases it O Stops the reactionarrow_forwardWhich of the following will not affect the reaction rate of a reaction? Increasing the surface area of a solid reactant. use of a catalyst Changing the pressure of the air in the room O increasing the concentration of the reactants All affect the reaction rate of a reaction.arrow_forward
- How does a catalyst influence the rate of a reaction?arrow_forwardReaction rate increased by an increase of surface area of a reactant. One way to increase surface area is to add catalyst crush solid reactant into smaller pieces increase reactant's temperature increase reactant's concentrationarrow_forwardWhich of the following is a definition of the activation energy of a reaction? O the difference in Gibbs free energy between the reactants and the transition state the difference in Gibbs free energy between the reactants and the intermediate O the difference in Gibbs free energy between the reactants and the product O the difference in Gibbs free energy between the transition state and the productarrow_forward
- If the energy of activation is lowered for a chemical reaction, which is true? OThe reaction proceeds faster The reaction must be exothermic OThe reaction does not occur O The reaction must be endothermic The reaction proceeds slowerarrow_forwardA chemical species that is not consumed during a chemical reaction and increases the rate of the reaction is referred to as a(n): A inhibitor. B catalyst. C spectator ion. D reactive intermediate.arrow_forwardEnzymes are important molecules in biochemistry that catalyze reactions. The energy diagram illustrates the difference between a catalyzed reaction and an uncatalyzed reaction. Label the energy diagram. 11 Answer Bank products reactants (substrate) activation energy uncatalyzed reaction reaction progress catalyzed reaction transition state free energy AG for reactionarrow_forward
- Which of the following will decrease the activation energy, Ea, of a reaction? O Increasing the reactant concentrations Removing the products as they form O Increasing the temperature O Adding a catalyst O Decreasing the pressure A Moving to another question will save this response. M esc 20 F3 F1 F2 F4 F5 @ #3 24 4 Q W R. Aarrow_forwardSketch an energy diagram graph representing an endothermic reaction, and label the following. a. Average energy of reactants b. Average energy of products c. Activation energy d. Amount of energy absorbed during the reactionarrow_forwardThe question is in the image.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY