
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
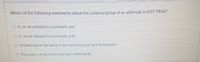
Transcribed Image Text:Which of the following statements about the carbonyl group of an aldehyde is NOTTRUE?
O It can be oxidized to a carboxylic acid.
O It can be reduced to a carboxylic acid.
O At least one of the atorms in the carbonyl group must be hydrogen.
O The carbon of the carbonyl group is electrophilic.

Transcribed Image Text:A fellow lab student is attempting to identify his unknown organic compound by using the Tollens' test. He
adds Tollens' reagent to a sample of benzaldehyde and to his unknown. You notice that neither his
benzaldehyde or unknown sámple solution formed a silver precipitate. The student confidently proclaims
that his unknown must not be an aldehyde. Is he correct?
Yes, because his unknown solution failed to form a silver precipitate that indicates the presence of an aldehyde.
O Yes, because the Tollens' test only forms a silver precipitate in the presence of an aromatic aldehyde.
O No, because his positive control did not form a silver precipitate, therefore he should gently heat the samples to verify
the result.
No, because the Tollens' test only forms a silver precipitate in the presence of a methyl ketone.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the IUPAC name for the organic compound formed when 1-propanol is dehydrated in the presence of an acid at 140 degrees Celsius.arrow_forwardWhich of the given compounds is/are hemiacetal, acetal, hemiketal and ketal?arrow_forwardWhich functional groups are absent in the following molecule? H ether ester aldehyde carboxylic acid ketonearrow_forward
- The following molecule is a :ÓH 2° alcohol 1° amine 1° alcohol 3° amine 3° alcohol 2° aminearrow_forwardDraw the skeletal structure for the product that forms when the alcohol 2,2-dimethyl-1-pentanol is oxidized. OHarrow_forwardWhich of the statements are true? 1. Aldehydes readily undergo oxidation to carboxylic acids 2. Propanone and acetone are two names for the same compound 3. The benedicts test distinguishes between aldehydes and ketonesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY