
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
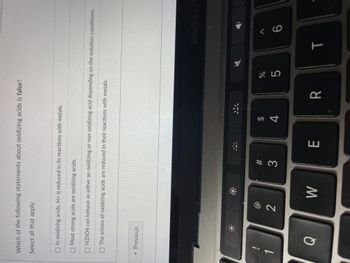
Transcribed Image Text:Which of the following statements about oxidizing acids is false?
Select all that apply.
In oxidizing acids, H+ is reduced in its reactions with metals.
Most strong acids are oxidizing acids.
O H2SO4 can behave as either an oxidizing or non oxidizing acid depending on the solution conditions.
The anions of oxidizing acids are reduced in their reactions with metals.
<< Previous
!
Q
8 N
2
W
#3
E
$
4
र
R
07 20
%
5
MacBook Pr
T
< 6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use Scenario 8.3 to answer the following question.The calculated mass of sodium bicarbonate in the antacid tablet is:arrow_forwardThe acid H2CO3 decomposes to some extent in water to produce CO2 gas. What should you expect to observe when the ingredients in an antacid react with HCl to produce H2CO3?arrow_forwardIn sediments and waterlogged soil, dissolved O2 concentrations are so low that the microorganisms living there must rely on other sources of oxygen for respiration. Some bacteria can extract the oxygen in sulfate ions, reducing the sulfur in them to hydrogen sulfide gas and giving the sediments or soil a distinctive rotten-egg odor.Write the net ionic equation for the reaction under acidic conditions (H3O+) that releases O2 from sulfate and forms hydrogen sulfide gas. Use water as the reactant in the half-reaction that describes the formation of oxygen.arrow_forward
- Which of these compounds are oxoacids (also known as oxyacids)? HF O hydrochloric acid chloric acid HCN H,PO, O Ba(OH), nitrous acidarrow_forwardQuestion 10 ef 27 Subm Predict the products (if any) that will be formed by the reaction below. If no reaction occurs, write NR after the reaction arrow. Al(s) + Na, So,(s) -arrow_forwardUsing your scientific knowledge, write a scientific explanation for what could be done to increase the forward reaction of the following equation: CaO(s) + H₂O(1) ⇒ Ca(OH)2(s) + heat CLAIM.arrow_forward
- When acids react with metals, which of the following products are normally formed? water salt hydrogen gas carbon dioxide none of the abovearrow_forward9. Complete and balance the following neutralization reaction H2SO4(aq) + NH,OH(aq) ing wollot orlarrow_forwardBaO2 + H2SO4 -> BaSO4 +H2O2 Determine the volume of 3.75 M sulfuric acid required to react with 17.6 grams of barium peroxide.arrow_forward
- You want to prepare barium sulfate, BaSO4, using n exchange reaction of some type. To do so you have the reagents shown from which the here from which to select the reactants Which two reagents coulde be used to form BaSO4 via an acid - base but not gas formiing reaction: here's the Reactants shown BaCL2, Ba(OH)2, Na2SO4, H2SO4, and BaCO3arrow_forwardComplete and balance HNO3(aq) + Mg(OH)2(s) -->arrow_forwardwrite a chemical and net ionic equation for the following elements that have reacted with water: Na Karrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY