
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
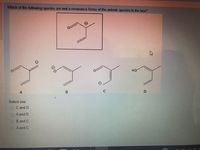
Transcribed Image Text:Which of the following species are not a resonance forms of the anionic species in the box?
HO
A
B
C
Select one:
O C and D
O A and D
O B and C
O A and C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CH3 CH3 HO: Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars, including charges where needed. The single bond is active by default. NN 1 [1] A 7 H 12D EXP. CONT ● Marvin JS by ChemAxon H C N O S CI Br - ס ד Farrow_forwardHow many resonance structures can be drawn for the AsO4 anionarrow_forward1. Which of the following elements has the lowest electronegativity? W, Ba, Pt Group of answer choices Ba Pt W 10. Arrange the following elements according to decreasing atomic radius: Al, P, Na, Cl 11. Which of the following elements has the lowest electronegativity? W, Ba, Ptarrow_forward
- Use ∆EN to determine the bond type (covalent, polar covalent, or ionic) in each of the following compounds which are either found on Mars, or needed for human colonization (reference table is in the package of reference tables)arrow_forwardWhich of the following species is/are not a resonance form(s) of the anionic species in the box? 32 32 3 O II and III OIII and IV OI and IV OI I and III O 10 O I II ◄ Previous III IV 39arrow_forwardQ50arrow_forward
- Consider the structure below, which is the best Lewis structure of the two resonance forms, A or B, and why? : F: Si-O: A. F: Si = 0: B. B because CO makes a stronger double bond A because Si-O is a longer bond. B because all atoms have a zero charge A because all atoms have a zero chargearrow_forwardWhich is the more polar bond in each of the following pairs? F―Cl or Br―Cl H―O or Se―H S―N or As―Harrow_forward+ 1 on CI- CI Q A Z @ 2 Click to edit molecule W S ●● command X Carbon tetrachloride, (CCI) is a solvent used in dry cleaning. Draw the Lewis structure of CCI and then determine the ideal bonding angle(s) for the CI-C-CI bond(s). www # 3 CJ Cl: . E D 8.0 F3 C $ 4 R JAN 25 A a FA FLO % 5 V T G Question 1 of 26 6 B FG Y H & 7 A) 90° B) 109.5° C) 120° D) 180° F7 לון U N 8 J DII FB I M ( 9 K F9 zoom O O H F10 L P command F11 { J F12 = ? option 1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY