
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
C revision
![Incorrect
Question 5
Which of the following solutions is most acidic?
a. [H] = 1 x 10-4
b. [H] = 1 x 10-14
c. [OH] = 1 × 10-3
d. pH = 10
e. pOH = 12
a
b](https://content.bartleby.com/qna-images/question/3f4c86b2-1247-4388-bf18-f90827f5643c/b81dd7dd-de48-4a62-8880-bc3b5b0c4b9e/ersia1h_thumbnail.jpeg)
Transcribed Image Text:Incorrect
Question 5
Which of the following solutions is most acidic?
a. [H] = 1 x 10-4
b. [H] = 1 x 10-14
c. [OH] = 1 × 10-3
d. pH = 10
e. pOH = 12
a
b
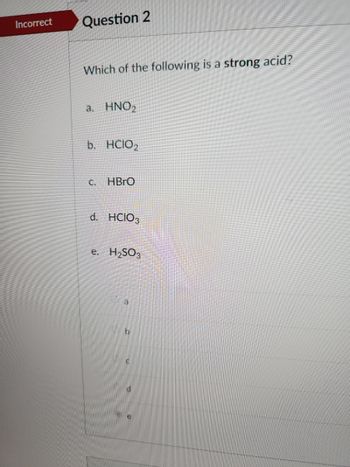
Transcribed Image Text:Incorrect Question 2
Which of the following is a strong acid?
a. HNO₂
b. HCIO₂
C.
HBrO
d. HCIO3
e. H₂SO3
Expert Solution
arrow_forward
Step 1
Dear student since you have posted multiple questions we will solve the first question for you if you want other question to be solved then kindly post them separately.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't use hend raitingarrow_forwardCarrow_forward← tab Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. s lock esc "-" ! 1 Q A :0 Select to Add Arrows 644 139.272 F1 H Select to Add Arrows @ 2 W :CI:Ⓒ 30: F2 # 3 31 80 F3 NOV 15 E S D $ 4 a F4 R LL % 5 tv♫♬ Ⓡ 0 F5 T 6 G Question 17 of 18 F6 A Y & 7 H 134044 F7 U Please select a d W 8 F8 J Aarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY