
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
5c ASALP
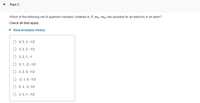
Transcribed Image Text:Part C
Which of the following set of quantum numbers (ordered n, l, me, mg) are possible for an electron in an atom?
Check all that apply.
• View Available Hint(s)
O 4, 2, 3, -1/2
O 3, 2, 2, -1/2
O 3, 2, 1, -1
О 3, 1, -2, -1/2
O 3, 2, 0, -1/2
O -2, 1, 0, -1/2
5, 3, -3, 1/2
O 3, 3, 1, -1/2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why is Cp > Cv?arrow_forwardGiven the Volumetric Analysis of a Natural Gas: CH4 = 67.3% Nitrogen = 9.6% O2 = 2.7% CO = 0.8% C2H6 = 15.4% CO2 = 1.8% H2 = 2.4% Calculate the A/F ratio by mass considering CO = 6.1% CO2. Note: Use four (4) decimal places in your solution and answer.arrow_forwardHow to find the specific volume of carbon dioxide at -40C for 800kPa and 1400kPaarrow_forward
- Ethylene (CH₂CH₂) is the starting point for a wide array of industrial chemical syntheses. For example, worldwide about 8.0 × 100 kg of polyethylene are made from ethylene each year, for use in everything from household plumbing to artificial joints. Natural sources of ethylene are entirely inadequate to meet world demand, so ethane (CH3CH3) from natural gas is "cracked" in refineries at high temperature in a kinetically complex reaction that produces ethylene gas and hydrogen gas. Suppose an engineer studying ethane cracking fills a 50.0 L reaction tank with 20.0 atm of ethane gas and raises the temperature to 600. °C. She believes K = 0.70 at this temperature. р alo Calculate the percent by mass of ethylene the engineer expects to find in the equilibrium gas mixture. Round your answer to 2 significant digits. Ar Note for advanced students: the engineer may be mistaken about the correct value of K, and the mass percent of ethylene you calculate may not be what she actually observes. %…arrow_forwardHow do you accomplish thisarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY