
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
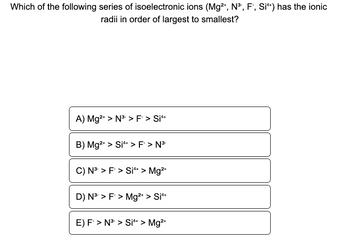
Transcribed Image Text:**Question:**
Which of the following series of isoelectronic ions (Mg²⁺, N³⁻, F⁻, Si⁴⁺) has the ionic radii in order of largest to smallest?
**Options:**
A) Mg²⁺ > N³⁻ > F⁻ > Si⁴⁺
B) Mg²⁺ > Si⁴⁺ > F⁻ > N³⁻
C) N³⁻ > F⁻ > Si⁴⁺ > Mg²⁺
D) N³⁻ > F⁻ > Mg²⁺ > Si⁴⁺
E) F⁻ > N³⁻ > Si⁴⁺ > Mg²⁺
**Explanations for Options:**
- **Option A** proposes that Mg²⁺ has the largest ionic radius, followed by N³⁻, F⁻, and Si⁴⁺ having the smallest.
- **Option B** orders Mg²⁺ as the largest, followed by Si⁴⁺, F⁻, and N³⁻ as the smallest.
- **Option C** suggests that N³⁻ has the largest ionic radius, with F⁻, Si⁴⁺, and Mg²⁺ decreasing in size respectively.
- **Option D** sequences N³⁻ as the largest, then F⁻, Mg²⁺, and Si⁴⁺ smallest.
- **Option E** presents F⁻ as having the largest radius, followed by N³⁻, Si⁴⁺, and Mg²⁺ being the smallest.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the largest ion (in volume) and the smallest ion in the following isoelectronic series: Al3+, Mg2+, Na+, F-, O2-, N3-arrow_forwardWhich of the following pairs of ions represent isoelectronic species? A) Mg²+ and O²- B) K+ and Li+ C) Ca²+ and F- D) Fe²+ and S²- E) Al³+ and Clarrow_forwardWhich of the following is an isoelectronic (have the same electron configuration) series? Group of answer choices O2-, F-, Ne, Na+ S, Cl, Ar, K B5-, Si4-, As3-, Te2- F-, Cl-, Br-, I- Si2-, P2-, S2-, Cl2-arrow_forward
- Two sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. S-> S+ + e- Ca-> Ca+ + e- He-> He+ + e- --------------- K-> K+ + e- Fr-> Fr+ + e- Si-> Si+ + e-arrow_forwardelement X has an electron affinity of 295.3 kJ/mol and the following ionization energies (in kJ/mol): IE1 = 1.008e3, IE2 = 1.846e3, IE3 = 3.20e3. In what column of the periodic table is this element probably found?arrow_forwardplease solve only question 7arrow_forward
- Order the elements below from smallest to largest 1st ionization energy. Mg, Cl, K, As _____ < ____< _____< ______,arrow_forwardplease solve only question 6arrow_forwardTwo sets of ionizations are shown in the tables below. Complete the tables by ordering each set of ionizations by increasing amount of energy required. In other words, for each set choose "1" next to the ionization that would require the least energy, "2" next to the ionization that would require the next least energy, and so on. ionization + Ge Ge + e Fr Fr + e + He He te energy required ? ? ? ionization + He → He + e + Cs Cs + e 0 0 + e X energy required ? ? Śarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY